The cGAS-STING-YY1 axis accelerates progression of neurodegeneration in a mouse model of Parkinson's disease via LCN2-dependent astrocyte senescence
- PMID: 37633968
- PMCID: PMC10589362
- DOI: 10.1038/s41418-023-01216-y
The cGAS-STING-YY1 axis accelerates progression of neurodegeneration in a mouse model of Parkinson's disease via LCN2-dependent astrocyte senescence
Abstract
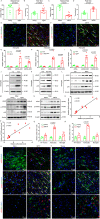
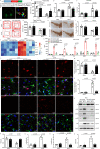
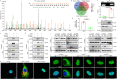
Recent studies provide clues that astrocyte senescence is correlated with Parkinson's disease (PD) progression, while little is known about the molecular basis for astrocyte senescence in PD. Here, we found that cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING) was upregulated in senescent astrocytes of PD and aged mice. Strikingly, deletion of astrocytic cGAS significantly prevented senescence of astrocytes and neurodegeneration. Furthermore, we identified LCN2 as the effector of cGAS-STING signal by RNA-Seq analysis. Genetic manipulation of LCN2 expression proved the regulation of cGAS-STING-LCN2 axis in astrocyte senescence. Additionally, YY1 was discovered as the transcription factor of LCN2 by chromatin immunoprecipitation. Binding of STING to YY1 impedes nuclear translocation of YY1. Herein, we determine the involvement of the cGAS-STING-YY1-LCN2 signaling cascade in the control of astrocyte senescence and PD progression. Together, this work fills the gap in our understanding of astrocyte senescence, and provides potential targets for delaying PD progression.
© 2023. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

