Netrin-1 blockade inhibits tumor associated Myeloid-derived suppressor cells, cancer stemness and alleviates resistance to chemotherapy and immune checkpoint inhibitor
- PMID: 37633969
- PMCID: PMC10589209
- DOI: 10.1038/s41418-023-01209-x
Netrin-1 blockade inhibits tumor associated Myeloid-derived suppressor cells, cancer stemness and alleviates resistance to chemotherapy and immune checkpoint inhibitor
Abstract
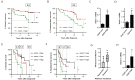
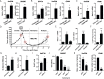
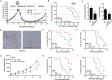
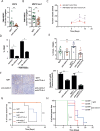
Drug resistance and cancer relapse represent significant therapeutic challenges after chemotherapy or immunotherapy, and a major limiting factor for long-term cancer survival. Netrin-1 was initially identified as a neuronal navigation cue but has more recently emerged as an interesting target for cancer therapy, which is currently clinically investigated. We show here that netrin-1 is an independent prognostic marker for clinical progression of breast and ovary cancers. Cancer stem cells (CSCs)/Tumor initiating cells (TICs) are hypothesized to be involved in clinical progression, tumor relapse and resistance. We found a significant correlation between netrin-1 expression and cancer stem cell (CSC) markers levels. We also show in different mice models of resistance to chemotherapies that netrin-1 interference using a therapeutic netrin-1 blocking antibody alleviates resistance to chemotherapy and triggers an efficient delay in tumor relapse and this effect is associated with CSCs loss. We also demonstrate that netrin-1 interference limits tumor resistance to immune checkpoint inhibitor and provide evidence linking this enhanced anti-tumor efficacy to a decreased recruitment of a subtype of myeloid-derived suppressor cells (MDSCs) called polymorphonuclear (PMN)-MDSCs. We have functionally demonstrated that these immune cells promote CSCs features and, consequently, resistance to anti-cancer treatments. Together, these data support the view of both a direct and indirect contribution of netrin-1 to cancer stemness and we propose that this may lead to therapeutic opportunities by combining conventional chemotherapies and immunotherapies with netrin-1 interfering drugs.
© 2023. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
BD, CV, JL, DN, DG, SD, AB and PM declare to have a conflict of interest as respectively employees (BD, DN, DG, CV, JL and SD) and shareholders (AB and PM) of NETRIS Pharma.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

