Critical limb-threatening ischaemia and microvascular transformation: clinical implications
- PMID: 37634134
- PMCID: PMC10821383
- DOI: 10.1093/eurheartj/ehad562
Critical limb-threatening ischaemia and microvascular transformation: clinical implications
Abstract
Background and aims: Clinical management of critical limb-threatening ischaemia (CLTI) is focused on prevention and treatment of atherosclerotic arterial occlusions. The role of microvascular pathology in disease progression is still largely unspecified and more importantly not utilized for treatment. The aim of this explorative study was to characterize the role of the microvasculature in CLTI pathology.
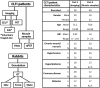
Methods: Clinical high-resolution imaging of CLTI patients (n = 50) and muscle samples from amputated CLTI limbs (n = 40) were used to describe microvascular pathology of CLTI at the level of resting muscle blood flow and microvascular structure, respectively. Furthermore, a chronic, low arterial driving pressure-simulating ischaemia model in rabbits (n = 24) was used together with adenoviral vascular endothelial growth factor A gene transfers to study the effect of microvascular alterations on muscle outcome.
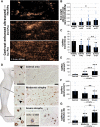
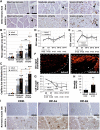
Results: Resting microvascular blood flow was not depleted but displayed decreased capillary transit time (P < .01) in CLTI muscles. Critical limb-threatening ischaemia muscle microvasculature also exhibited capillary enlargement (P < .001) and further arterialization along worsening of myofibre atrophy and detaching of capillaries from myofibres. Furthermore, CLTI-like capillary transformation was shown to worsen calf muscle force production (P < .05) and tissue outcome (P < .01) under chronic ischaemia in rabbits and in healthy, normal rabbit muscle.
Conclusions: These findings depict a progressive, hypoxia-driven transformation of the microvasculature in CLTI muscles, which pathologically alters blood flow dynamics and aggravates tissue damage under low arterial driving pressure. Hypoxia-driven capillary enlargement can be highly important for CLTI outcomes and should therefore be considered in further development of diagnostics and treatment of CLTI.
Keywords: Angiogenesis; Capillaries; Chronic limb-threatening ischaemia; Growth factor; Hypoxia; Ischaemia; Microvascular remodelling; Peripheral arterial disease; Vascular endothelial.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Comment in
-
A conundrum of arterialized capillaries and vascular dilation in chronic limb-threatening ischaemia.Eur Heart J. 2024 Jan 27;45(4):265-267. doi: 10.1093/eurheartj/ehad826. Eur Heart J. 2024. PMID: 38126898 Free PMC article. No abstract available.
References
-
- Aboyans V, Ricco J-B, Bartelink M-LEL, Björck M, Brodmann M, Cohnert T, et al. . 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. 10.1093/eurheartj/ehx095 - DOI - PubMed
-
- Criqui MH, Matsushita K, Aboyans V, Hess CN, Hicks CW, Kwan TW, et al. . Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American Heart Association. Circulation 2021;144:E171–91. 10.1161/CIR.0000000000001005 - DOI - PMC - PubMed
-
- Burchert W, Schellong S, van den Hoff J, Meyer GJ, Alexander K, Hundeshagen H. Oxygen-15-water PET assessment of muscular blood flow in peripheral vascular disease. J Nucl Med 1997;38:93–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

