Which severe COVID-19 patients could benefit from high dose dexamethasone? A Bayesian post-hoc reanalysis of the COVIDICUS randomized clinical trial
- PMID: 37634234
- PMCID: PMC10460760
- DOI: 10.1186/s13613-023-01168-z
Which severe COVID-19 patients could benefit from high dose dexamethasone? A Bayesian post-hoc reanalysis of the COVIDICUS randomized clinical trial
Erratum in
-
Correction: Which severe COVID-19 patients could benefit from high dose dexamethasone? A Bayesian post-hoc reanalysis of the COVIDICUS randomized clinical trial.Ann Intensive Care. 2023 Sep 29;13(1):95. doi: 10.1186/s13613-023-01194-x. Ann Intensive Care. 2023. PMID: 37773550 Free PMC article. No abstract available.
Abstract
Background: The respective benefits of high and low doses of dexamethasone (DXM) in patients with severe acute respiratory syndrome coronavirus 2 (SARS-Cov2) and acute respiratory failure (ARF) are controversial, with two large triple-blind RCTs reaching very important difference in the effect-size. In the COVIDICUS trial, no evidence of additional benefit of high-dose dexamethasone (DXM20) was found. We aimed to explore whether some specific patient phenotypes could benefit from DXM20 compared to the standard of care 6 mg dose of DXM (DXMSoC).
Methods: We performed a post hoc exploratory Bayesian analysis of 473 patients who received either DXMSoc or DXM20 in the COVIDICUS trial. The outcome was the 60 day mortality rate of DXM20 over DXMSoC, with treatment effect measured on the hazard ratio (HR) estimated from Cox model. Bayesian analyses allowed to compute the posterior probability of a more than trivial benefit (HR < 0.95), and that of a potential harm (HR > 1.05). Bayesian measures of interaction then quantified the probability of interaction (Pr Interact) that the HR of death differed across the subsets by 20%. Primary analyses used noninformative priors, centred on HR = 1.00. Sensitivity analyses used sceptical and enthusiastic priors, based on null (HR = 1.00) or benefit (HR = 0.95) effects.
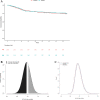
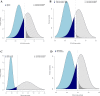
Results: Overall, the posterior probability of a more than trivial benefit and potential harm was 29.0 and 51.1%, respectively. There was some evidence of treatment by subset interaction (i) according to age (Pr Interact, 84%), with a 86.5% probability of benefit in patients aged below 70 compared to 22% in those aged above 70; (ii) according to the time since symptoms onset (Pr Interact, 99%), with a 99.9% probability of a more than trivial benefit when lower than 7 days compared to a < 0.1% probability when delayed by 7 days or more; and (iii) according to use of remdesivir (Pr Interact, 91%), with a 90.1% probability of benefit in patients receiving remdesivir compared to 19.1% in those who did not.
Conclusions: In this exploratory post hoc Bayesian analysis, compared with standard-of-care DXM, high-dose DXM may benefit patients aged less than 70 years with severe ARF that occurred less than 7 days after symptoms onset. The use of remdesivir may also favour the benefit of DXM20. Further analysis is needed to confirm these findings.
Trial registration: NCT04344730, date of registration April 14, 2020 ( https://clinicaltrials.gov/ct2/show/NCT04344730?term=NCT04344730&draw=2&rank=1 ); EudraCT: 2020-001457-43 ( https://www.clinicaltrialsregister.eu/ctr-search/search?query=2020-001457-43 ).
Keywords: ARDS; Acute respiratory failure; Bayesian analysis; Critical care; Dose; Interaction; Remdesivir; SARS-Cov-2; Sepsis; Steroids.
© 2023. La Société de Réanimation de Langue Francaise = The French Society of Intensive Care (SRLF).
Conflict of interest statement
J.F. Timsit reports lecture fees from Merck, Pfizer, Shionogi, Gilead, and Thermo Fisher and advisory board participation for Merck, Pfizer, Shionogi, Beckton-Dickinson, Aspen, and Gilead unrelated to the submitted work.
Figures

References
-
- Bouadma L, Mekontso-Dessap A, Burdet C, et al. High-dose dexamethasone and oxygen support strategies in intensive care unit patients with severe COVID-19 acute hypoxemic respiratory failure: the COVIDICUS randomized clinical trial. JAMA Intern Med. 2022 doi: 10.1001/jamainternmed.2022.2168. - DOI - PMC - PubMed
-
- Taboada M, Rodríguez N, Varela PM, et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2022;60(2):2102518. doi: 10.1183/13993003.02518-2021. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

