Timing of maternal isoniazid preventive therapy on tuberculosis infection among infants exposed to HIV in low-income and middle-income settings: a secondary analysis of the TB APPRISE trial
- PMID: 37634517
- PMCID: PMC10883460
- DOI: 10.1016/S2352-4642(23)00174-8
Timing of maternal isoniazid preventive therapy on tuberculosis infection among infants exposed to HIV in low-income and middle-income settings: a secondary analysis of the TB APPRISE trial
Abstract
Background: Infants born to women with HIV in settings with a high tuberculosis burden are at risk of tuberculosis infection and rapid progression to active disease. Maternal isoniazid preventive therapy might mitigate this risk, but optimal timing of therapy remains unclear. The TB APPRISE trial showed that initiation of isoniazid during pregnancy resulted in more frequent adverse pregnancy outcomes than when initiated postpartum. We aimed to determine the proportion of infants testing positive for tuberculosis infection born to mothers who initiated isoniazid therapy antepartum compared with postpartum using two commonly used tests, the test agreement, and predictors of test positivity.
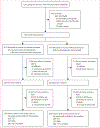
Methods: TB APPRISE was a randomised, double-blind, placebo-controlled, non-inferiority trial done at 13 study sites across eight countries (Botswana, Haiti, India, South Africa, Tanzania, Thailand, Uganda, and Zimbabwe). Pregnant women with HIV on antiretroviral therapy were randomly assigned to receive immediate isoniazid preventive therapy (28 weeks isoniazid [300 mg daily], then placebo until week 40 after delivery) or deferred treatment (placebo until week 12 after delivery, then isoniazid [300 mg daily] for 28 weeks). Mother-infant pairs were followed up until 48 weeks after delivery. We included all liveborn infants with a tuberculin skin test or interferon-γ release assay (IGRA) at 44 weeks. The outcomes assessed in this secondary analysis were tuberculosis test positivity by study group, test agreement, and predictors of test positivity. This study was registered with ClinicalTrials.gov, NCT01494038.
Findings: Between Aug 19, 2014, and April 4, 2016, 956 mothers were randomly assigned, and 749 mother-child pairs were included in this secondary analysis. Of 749 infants, 694 (93%) received Bacille Calmette-Guérin (BCG) vaccination, 675 (90%) were born to mothers who had completed isoniazid treatment, 20 (3%) were exposed to tuberculosis, seven (1%) became HIV positive, and one (<1%) developed probable tuberculosis. 43 (6%; 95% CI 4-8]) of 732 infants had a positive IGRA test result and 55 (8%; 6-10) of 727 infants had a positive tuberculin skin test result. Test positivity did not differ by study group (p=0·88 for IGRA; p=0·44 for tuberculin skin test). Test agreement was poor (κ=0·107 [95% CI 0·002-0·212]). Infant tuberculin skin test positivity was associated with breastfeeding (adjusted odds ratio 6·63 [95% CI 1·57-27·9]), BCG vaccination (4·97 [1·50-16·43]), and maternal tuberculin skin test positivity at delivery (3·28 [1·70-6·33]); IGRA positivity was associated with female sex (2·09 [1·06-4·14]).
Interpretation: Deferral of maternal isoniazid preventive therapy to early postpartum had no effect on infant tuberculosis acquisition in our trial population, regardless of the diagnostic test used; however, tuberculosis test agreement is poor during infancy.
Funding: US National Institutes of Health.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

References
-
- WHO. Global tuberculosis report Geneva: World Health Organization, 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa... (accessed April 23, 2023).
-
- Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012; 367: 348–61. - PubMed
-
- Reid MJA, Arinaminpathy N, Bloom A, et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 2019; 393: 1331–84. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

