Neuroimmune interplay during type 2 inflammation: Symptoms, mechanisms, and therapeutic targets in atopic diseases
- PMID: 37634890
- PMCID: PMC11215634
- DOI: 10.1016/j.jaci.2023.08.017
Neuroimmune interplay during type 2 inflammation: Symptoms, mechanisms, and therapeutic targets in atopic diseases
Abstract
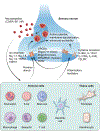
Type 2 inflammation is characterized by overexpression and heightened activity of type 2 cytokines, mediators, and cells that drive neuroimmune activation and sensitization to previously subthreshold stimuli. The consequences of altered neuroimmune activity differ by tissue type and disease; they include skin inflammation, sensitization to pruritogens, and itch amplification in atopic dermatitis and prurigo nodularis; airway inflammation and/or hyperresponsiveness, loss of expiratory volume, airflow obstruction and increased mucus production in asthma; loss of sense of smell in chronic rhinosinusitis with nasal polyps; and dysphagia in eosinophilic esophagitis. We describe the neuroimmune interactions that underlie the various sensory and autonomic pathologies in type 2 inflammatory diseases and present recent advances in targeted treatment approaches to reduce type 2 inflammation and its associated symptoms in these diseases. Further research is needed to better understand the neuroimmune mechanisms that underlie chronic, sustained inflammation and its related sensory pathologies in diseases associated with type 2 inflammation.
Keywords: Type 2 inflammation; asthma; atopic dermatitis; chronic rhinosinusitis with nasal polyposis; cytokines; eosinophilic esophagitis; neuroimmune; neuropeptides; prurigo nodularis; sensory neurons.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest:
B. S. Kim is founder of Klirna Biotech; in addition, he has served as a consultant for 23andMe, AbbVie, Almirall, Amagma, Arcutis Biotherapeutics, Arena Pharmaceuticals, Argenx, AstraZeneca, Bellus Health, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Clexio Biosciences, Cymabay Therapeutics, Daewoong Pharmaceutical, Eli Lilly, Escient Pharmaceuticals, Evommune, FIDE, Galderma, Genentech, GSK, Granular Therapeutics, IQVIA, Incyte, Innovaderm Research, Janssen, Kiniksa, LEO Pharma, Maruho, Medicxi, Menlo Therapeutics, Novartis, OM Pharma, Pfizer, Recens Medical, Regeneron Pharmaceuticals Inc, Sanofi, Septerna, Shaperon, Third Harmonic Bio, Vial, and WebMD; he has stock in Abrax Japan, KliRNA Biotech, Locus Biosciences, and RecensMedical; and he holds a patent for the use of JAK1 inhibitors for chronic pruritus. M. E. Rothenberg is a consultant for Allakos, AstraZeneca, Bristol Myers Squibb, Celldex Therapeutics, ClostraBio, Ellodi Pharma, GSK, Guidepoint, Nextstone One, PulmOne, Revolo Biotherapeutics, Sanofi-Regeneron Pharmaceuticals Inc, Serpin Pharma, and Spoon Guru; in addition, he has an equity interest in the first 7 of the aforementioned companies; receives royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate; and is an inventor of patents owned by Cincinnati Children’s Hospital. C. Bachert is a consultant for GSK, Mensarini, Novartis, and Sanofi. D. Artis has contributed to scientific advisory boards at FARE, the KRF, Pfizer, and Takeda; research in the Artis laboratory is supported by the National Institutes of Health (grants DK126871, AI151599, AI095466, AI095608, AI142213, AR070116, AI172027, and DK132244), the Crohn’s and Colitis Foundation, Cure for IBD, the Jill Roberts Institute, the Sanders Family, and the Rosanne H. Silbermann Foundation. R. Zaheer and P. Rowe are employees of and may hold stock and/or stock options in Sanofi. Y. Deniz and S. Cyr are employees and shareholders at Regeneron Pharmaceuticals Inc. The remaining author declares that he has no relevant conflicts of interest.
Figures
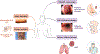

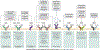
References
-
- Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016;15:35–50. - PubMed
-
- Gandhi NA, Pirozzi G, Graham NM. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol 2017;13:425–37. - PubMed
-
- Kopp EB, Agaronyan K, Licona-Limon I, Nish SA, Medzhitov R. Modes of type 2 immune response initiation. Immunity 2023;56:667–94. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

