Generation of Human Endometrial Assembloids with a Luminal Epithelium using Air-Liquid Interface Culture Methods
- PMID: 37635169
- PMCID: PMC10602567
- DOI: 10.1002/advs.202301868
Generation of Human Endometrial Assembloids with a Luminal Epithelium using Air-Liquid Interface Culture Methods
Abstract
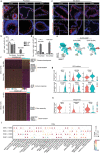
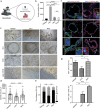
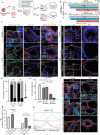
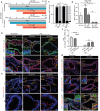
The endometrial lining of the uterus is essential for women's reproductive health and consists of several different types of epithelial and stromal cells. Although models such as gland-like structures (GLSs) and endometrial assembloids (EnAos) are successfully established, they lack an intact luminal epithelium, which makes it difficult to recapitulate endometrial receptivity. Here, a novel EnAo model (ALI-EnAo) is developed by combining endometrial epithelial cells (EnECs) and stromal cells (EnSCs) and using an improved matrix and air-liquid interface (ALI) culture method. ALI-EnAos exhibit intact EnSCs and glandular and luminal epithelia, which recapitulates human endometrium anatomy, cell composition, hormone-induced menstrual cycle changes, gene expression profiles, and dynamic ciliogenesis. The model suggests that EnSCs, together with the extracellular matrix and ALI culture conditions, contribute to EnAo phenotypes and characteristics reflective of the endometrial menstrual cycle. This enables to transcriptionally define endometrial cell subpopulations. It anticipates that ALI-EnAos will facilitate studies on embryo implantation, and endometrial growth, differentiation, and disease.
Keywords: air-liquid interface; endometrial assembloids; luminal epithelium; organoid.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids.Elife. 2021 Sep 6;10:e69603. doi: 10.7554/eLife.69603. Elife. 2021. PMID: 34487490 Free PMC article.
-
Tripeptidyl peptidase I promotes human endometrial epithelial cell adhesive capacity implying a role in receptivity.Reprod Biol Endocrinol. 2020 Dec 14;18(1):124. doi: 10.1186/s12958-020-00682-0. Reprod Biol Endocrinol. 2020. PMID: 33317560 Free PMC article.
-
Luminal and Glandular Epithelial Cells from the Porcine Endometrium maintain Cell Type-Specific Marker Gene Expression in Air-Liquid Interface Culture.Stem Cell Rev Rep. 2022 Dec;18(8):2928-2938. doi: 10.1007/s12015-022-10410-3. Epub 2022 Jul 18. Stem Cell Rev Rep. 2022. PMID: 35849251 Free PMC article.
-
How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation.Cells. 2021 Aug 6;10(8):2008. doi: 10.3390/cells10082008. Cells. 2021. PMID: 34440776 Free PMC article. Review.
-
FoxO1 is a cell-specific core transcription factor for endometrial remodeling and homeostasis during menstrual cycle and early pregnancy.Hum Reprod Update. 2021 Apr 21;27(3):570-583. doi: 10.1093/humupd/dmaa060. Hum Reprod Update. 2021. PMID: 33434267 Review.
Cited by
-
Organoids: development and applications in disease models, drug discovery, precision medicine, and regenerative medicine.MedComm (2020). 2024 Sep 21;5(10):e735. doi: 10.1002/mco2.735. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39309690 Free PMC article. Review.
-
Bioengineering approaches for the endometrial research and application.Mater Today Bio. 2024 Apr 3;26:101045. doi: 10.1016/j.mtbio.2024.101045. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38600921 Free PMC article. Review.
-
The long road of drug development for endometriosis - Pains, gains, and hopes.J Control Release. 2024 Dec;376:429-440. doi: 10.1016/j.jconrel.2024.10.036. Epub 2024 Oct 24. J Control Release. 2024. PMID: 39427778 Free PMC article. Review.
-
Enhanced myofibroblast differentiation of eMSCs in intrauterine adhesions.Stem Cell Res Ther. 2025 Feb 4;16(1):35. doi: 10.1186/s13287-025-04183-y. Stem Cell Res Ther. 2025. PMID: 39901307 Free PMC article.
-
Basic Research Advances in China on Embryo Implantation, Placentation, and Parturition.Matern Fetal Med. 2024 Jan 15;6(1):37-49. doi: 10.1097/FM9.0000000000000210. eCollection 2024 Jan. Matern Fetal Med. 2024. PMID: 40406740 Free PMC article.
References
-
- a) Lancaster M. A., Knoblich J. A., Science 2014, 345, 1247125; - PubMed
- b) Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., Sasai Y., Cell Stem Cell 2012, 10, 771; - PubMed
- c) Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K., Hoskins E. E., Kalinichenko V. V., Wells S. I., Zorn A. M., Shroyer N. F., Wells J. M., Nature 2011, 470, 105. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 2022YFA1103100/National Key Research and Development Program of China
- 2021YFA0805700/National Key Research and Development Program of China
- 32130034/National Natural Science Foundation of China
- 82192874/National Natural Science Foundation of China
- 202001BC070001/Major Basic Research Project of Science and Technology of Yunnan
- 2019FY002/Major Basic Research Project of Science and Technology of Yunnan
- YNWR-YLXZ-2020-015/Major Basic Research Project of Science and Technology of Yunnan
- Spring City Plan, the High-level Talent Promotion and Training Project of Kunming
- Reproduction and Obstetrics and Gynecology Clinical Medical Center of Yunnan
LinkOut - more resources
Full Text Sources
