Pharmacological treatment of gastro-oesophageal reflux in children
- PMID: 37635269
- PMCID: PMC10443045
- DOI: 10.1002/14651858.CD008550.pub3
Pharmacological treatment of gastro-oesophageal reflux in children
Abstract
Background: Gastro-oesophageal reflux (GOR) is characterised by the regurgitation of gastric contents into the oesophagus. GOR is a common presentation in infancy, both in primary and secondary care, affecting approximately 50% of infants under three months old. The natural history of GOR in infancy is generally of a self-limiting condition that improves with age, but older children and children with co-existing medical conditions can have more protracted symptoms. The distinction between gastro-oesophageal reflux disease (GORD) and GOR is debated. Current National Institute of Health and Care Excellence (NICE) guidelines define GORD as GOR causing symptoms severe enough to merit treatment. This is an update of a review first published in 2014.
Objectives: To assess the effects of pharmacological treatments for GOR in infants and children.
Search methods: For this update, we searched CENTRAL, MEDLINE, Embase, and Web of Science up to 17 September 2022. We also searched for ongoing trials in clinical trials registries, contacted experts in the field, and searched the reference lists of trials and reviews for any additional trials.
Selection criteria: We included randomised controlled trials (RCTs) that compared any currently-available pharmacological treatment for GOR in children with placebo or another medication. We excluded studies assessing dietary management of GORD and studies of thickened feeds. We included studies in infants and children up to 16 years old.
Data collection and analysis: We used standard methodology expected by Cochrane.
Main results: We included 36 RCTs involving 2251 children and infants. We were able to extract summary data from 14 RCTs; the remaining trials had insufficient data for extraction. We were unable to pool results in a meta-analysis due to methodological differences in the included studies (including heterogeneous outcomes, study populations, and study design). We present the results in two groups by age: infants up to 12 months old, and children aged 12 months to 16 years old. Infants Omeprazole versus placebo: there is no clear effect on symptoms from omeprazole. One study (30 infants; very low-certainty evidence) showed cry/fuss time in infants aged three to 12 months had altered from 246 ± 105 minutes/day at baseline (mean +/- standard deviation (SD)) to 191 ± 120 minutes/day in the omeprazole group and from 287 ± 132 minutes/day to 201 ± 100 minutes/day in the placebo group (mean difference (MD) 10 minutes/day lower (95% confidence interval (CI) -89.1 to 69.1)). The reflux index changed in the omeprazole group from 9.9 ± 5.8% in 24 hours to 1.0 ± 1.3% and in the placebo group from 7.2 ± 6.0% to 5.3 ± 4.9% in 24 hours (MD 7% lower, 95% CI -4.7 to -9.3). Omeprazole versus ranitidine: one study (76 infants; very low-certainty evidence) showed omeprazole may or may not provide symptomatic benefit equivalent to ranitidine. Symptom scores in the omeprazole group changed from 51.9 ± 5.4 to 2.4 ± 1.2, and in the ranitidine group from 47 ± 5.6 to 2.5 ± 0.6 after two weeks: MD -4.97 (95% CI -7.33 to -2.61). Esomeprazole versus placebo: esomeprazole appeared to show no additional reduction in the number of GORD symptoms compared to placebo (1 study, 52 neonates; very low-certainty evidence): both the esomeprazole group (184.7 ± 78.5 to 156.7 ± 75.1) and placebo group (183.1 ± 77.5 to 158.3 ± 75.9) improved: MD -3.2 (95% CI -4.6 to -1.8). Children Proton pump inhibitors (PPIs) at different doses may provide little to no symptomatic and endoscopic benefit. Rabeprazole given at different doses (0.5 mg/kg and 1 mg/kg) may provide similar symptom improvement (127 children in total; very low-certainty evidence). In the lower-dose group (0.5 mg/kg), symptom scores improved in both a low-weight group of children (< 15 kg) (mean -10.6 ± SD 11.13) and a high-weight group of children (> 15 kg) (mean -13.6 ± 13.1). In the higher-dose groups (1 mg/kg), scores improved in the low-weight (-9 ± 11.2) and higher-weight groups (-8.3 ± 9.2). For the higher-weight group, symptom score mean difference between the two different dosing regimens was 2.3 (95% CI -2 to 6.6), and for the lower-weight group, symptom score MD was 4.6 (95% CI -2.9 to 12). Pantoprazole: pantoprazole may or may not improve symptom scores at 0.3 mg/kg, 0.6 mg/kg, and 1.2 mg/kg pantoprazole in children aged one to five years by week eight, with no difference between 0.3 mg/kg and 1.2 mg/kg dosing (0.3 mg/kg mean -2.4 ± 1.7; 1.2 mg/kg -1.7 ± 1.2: MD 0.7 (95% CI -0.4 to 1.8)) (one study, 60 children; very low-certainty evidence). There were insufficient summary data to assess other medications.
Authors' conclusions: There is very low-certainty evidence about symptom improvements and changes in pH indices for infants. There are no summary data for endoscopic changes. Medications may or may not provide a benefit (based on very low-certainty evidence) for infants whose symptoms remain bothersome, despite nonmedical interventions or parental reassurance. If a medication is required, there is no clear evidence based on summary data for omeprazole, esomeprazole (in neonates), H₂antagonists, and alginates for symptom improvements (very low-certainty evidence). Further studies with longer follow-up are needed. In older children with GORD, in studies with summary data extracted, there is very low-certainty evidence that PPIs (rabeprazole and pantoprazole) may or may not improve GORD outcomes. No robust data exist for other medications. Further RCT evidence is required in all areas, including subgroups (preterm babies and children with neurodisabilities).
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
There are no authors' conflicts of interest.
A previous review of the medical treatment of gastro‐oesophageal reflux was completed for 'Pediatric Drugs' (publishers: 'Adis') and published in early 2009. However that article is substantially different from the Cochrane review. The Pediatric Drugs article was not funded.
Figures
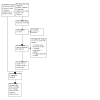
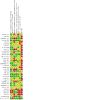
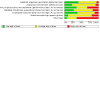







Update of
-
Pharmacological treatment of children with gastro-oesophageal reflux.Cochrane Database Syst Rev. 2014 Nov 24;2014(11):CD008550. doi: 10.1002/14651858.CD008550.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2023 Aug 22;8:CD008550. doi: 10.1002/14651858.CD008550.pub3. PMID: 25419906 Free PMC article. Updated.
References
References to studies included in this review
Azizollahi 2016 {published data only}
Baker 2010 {published data only}
-
- Baker R, Tsou VM, Tung J, Baker SS, Li H, Wang W, et al. Clinical results from a randomised, double-blind, dose-ranging study of pantoprazole in children aged 1 through 5 years with symptomatic histologic or erosive esophagitis. Clinical Pediatrics 2010;49(9):852-65. - PubMed
Baldassarre 2020 {published data only}
Ballengee 2018 {published data only}
-
- Ballengee CR, Davalian F, Conaway MR, Sauer CG, Kaufman DA. Erythromycin and reflux events in premature neonates: a randomized clinical trial. Journal of Pediatric Gastroenterology and Nutrition 2018;67(6):720-25. - PubMed
Bines 1992 {published data only}
-
- Bines JE, Quinlan JE, Treves S, Kleinman RE, Winter HS. Efficacy of domperidone in infants and children with gastroesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition 1992;14(4):400-5. - PubMed
Borrelli 2002 {published data only}
-
- Borrelli O, Rea P, Bueno De Mesquita M, Ambrosini A, Mancini V, Di Nardo G, et al. Efficacy of combined administration of an alginate formulation (Gaviscon) and Lansoprazole for children with gastroesophageal reflux. Italian Journal of Pediatrics 2002;28(4):304-9.
Buts 1987 {published data only}
-
- Buts JP, Barudi C, Otte JB. Double-blind controlled study on the efficacy of sodium alginate (Gaviscon) in reducing gastroesophageal reflux assessed by 24 h continuous pH monitoring in infants and children. European Journal of Pediatrics 1987;146(2):156-8. - PubMed
Carroccio 1994 {published data only}
-
- Carroccio A, Iacono, Montalto G, Cavataio F, Soresi M, Notarbartolo A. Domperidone plus magnesium hydroxide and aluminium hydroxide: a valid therapy in children with gastroesophageal reflux. Scandinavian Journal of Gastroenterology 1994;29(4):300-4. - PubMed
Cresi 2008 {published data only}
-
- Cresi F, Marinaccio C, Russo MC, Miniero R, Silvestro L. Short-term effect of domperidone on gastroesophageal reflux in newborns assessed by combined intraluminal impedance and pH monitoring. Journal of Perinatology 2008;28:766-70. - PubMed
Cucchiara 1984 {published data only}
Cucchiara 1993 {published data only}
Davidson 2013 {published data only}
-
- Davidson G, Wenzl TG, Thomson M, Omari T, Barker P, Lundborg P, et al. Efficacy and safety of once-daily esomeprazole for the treatment of gastroesophageal reflux disease in neonatal patients. Journal of Pediatrics 2013;163(3):692-8. - PubMed
Del Buono 2005 {published data only}
Famouri 2017 {published data only}
-
- Famouri F, Zibanejad N, Kabiri P, Kelishad R. Comparison of hypoallergenic diet vs. ranitidine in treatment of gastroesophageal reflux disease of infants: a randomized clinical trial. Iranian Journal of Pediatrics 2017;27(4):e5343.
Forbes 1986 {published data only}
-
- Forbes D, Hodgson M, Hill R. The effects of gaviscon and metoclopramide in gastroesophageal reflux in children. Journal of Pediatric Gastroenterology and Nutrition 1986;5(4):556-9. - PubMed
Gilger 2006 {published data only}
-
- Gilger M, Tolia V, Vandenplas Y, Youssef N, Traxler B, Illueca M. Safety and tolerability of esomeprazole in children with gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2006;43(4):E20-21. - PubMed
-
- Gilger MA, Tolia V, Vandenplas Y, Youssef NN, Traxler B, Illueca M. Safety and tolerability of esomeprazole in children with gastro-esophageal reflux disease. Gastroenterology 2015;46(5):S16-23. - PubMed
Gunesekaran 2003 {published data only}
-
- Gunasekaran T, Gupta S, Gremse D, Karol M, Pan W-J, Chin Y-L, et al. Lansoprazole in adolescents with gastroesophageal reflux disease: pharmacokinetics, pharmacodynamics, symptom relief efficacy, and tolerability. Journal of Pediatric Gastroenterology and Nutrition 2002;35(4):S327-35. - PubMed
Haddad 2013 {published data only}
-
- Haddad I, Kierkus J, Tron E, Ulmer A, Hu P, Sloan S, et al. Efficacy and safety of rabeprazole in children (1-11 years) with gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2013;57(6):798-807. - PubMed
Hussain 2014 {published data only}
-
- Hussain S, Kierkus J, Hu P, Hoffman D, Lekich R, Sloan S, et al. Safety and efficacy of delayed release rabeprazole in 1- to 11-month-old infants with symptomatic GERD. Journal of Pediatric Gastroenterology and Nutrition 2014;58(2):226-36. - PubMed
Kierkus 2011 {published data only}
-
- Kierkus J, Furmaga-Jablonska W, Sullivan JE, David ES, Stewart DL, Rath N, et al. Pharmacodynamics and safety of pantoprazole in neonates, preterm infants, and infants aged 1-11 months with a clinical diagnosis of gastroesophageal reflux disease. Digestive Diseases and Sciences 2011;56:425-34. - PubMed
Loots 2014 {published data only}
-
- Loots C, Kritas S, Wijk M, McCall L, Peeters L, Lewindon P, et al. Body positioning and medical therapy for infantile gastroesophageal reflux symptoms. Journal of Pediatric Gastroenterology and Nutrition 2014;59(2):237-43. - PubMed
Miller 1999 {published data only}
-
- Miller S. Comparison of the efficacy and safety of a new aluminum-free paediatric alginate preparation and placebo in infants with recurrent gastro-oesophageal reflux. Current Medical Research and Opinion 1999;15(3):160-8. - PubMed
Moore 2003 {published data only}
-
- Moore DJ, Tao BS, Lines DR, Hirte C, Heddle ML, Davidson GP. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. Journal of Pediatrics 2003;143(2):219-23. - PubMed
Naeimi 2019 {published data only}
-
- Naeimi M, Kianifar H, Memariani Z, Kamalinejad M, Bijani A, Saghebi R, et al. Comparison of the efficacy of ranitidine and quince syrup on gastroesophageal reflux disease in children. Complementary Therapies in Medicine 2019;45:215-21. - PubMed
Omari 2006 {published data only}
-
- Omari TI, Benninga MA, Sansom L, Butler RN, Dent J, Davidson GP. Effect of baclofen on esophagogastric motility and gastroesophageal reflux in children with gastroesophageal reflux disease: a randomized controlled trial. Journal of Pediatrics 2006;149(4):468-74. - PubMed
Omari 2007 {published data only}
-
- Omari T, Davidson G, Bondarov P, Naucler E, Nilsson C, Lundborg P. Pharmacokinetics and acid-suppressive effects of esomeprazole in infants 1-24 months old with symptoms of gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2007;45(5):530-7. [MEDLINE: Published in abstract form in JPGN:2006: 42: E1-110] - PubMed
-
- Omari T, Davidson G, Bondarov P, Naucler E, Nilsson C, Lundborg P. Pharmacokinetics and acid-suppressive effects of esomeprazole in infants 1-24 months old with symptoms of gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2015;60:S2-S8. - PubMed
Orenstein 2002 {published data only}
-
- Orenstein SR, Shalaby TM, Devandry SN, Liacouras CA, Czinn SJ, Dice JE, et al. Famotidine for infant gastro-oesophageal reflux: a multi-centre, randomized, placebo-controlled, withdrawal trial. Alimentary Pharmacology and Therapeutics 2003;17(9):1097-107. - PubMed
Orenstein 2008 {published data only}
-
- Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. Journal of Pediatrics 2009;154(4):514-20. - PubMed
Pfefferkorn 2006 {published data only}
-
- Pfefferkorn MD, Croffie JM, Gupta SK, Molleston JP, Eckert GJ, Corkins MR, et al. Nocturnal acid breakthrough in children with reflux esophagitis taking proton pump inhibitors. Journal of Pediatric Gastroenterology and Nutrition 2006;42(2):160-5. - PubMed
Simeone 1997 {published data only}
-
- Simeone D, Caria MC, Miele E, Staiano A. Treatment of childhood peptic esophagitis: a double-blind placebo-controlled trial of nizatidine. Journal of Pediatric Gastroenterology and Nutrition 1997;25(1):51-5. - PubMed
Tolia 2006 {published data only}
-
- Tolia V, Bishop PR, Tsou VM, Gremse D, Soffer EF, Comer GM, et al. Multicenter, randomized, double-blind study comparing 10, 20 and 40 mg pantoprazole in children (5-11 years) with symptomatic gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2006;42(4):384-91. - PubMed
Tolia 2010a {published data only}
-
- Tolia V, Gilger MA, Barker PN, Illueca M. Healing of erosive esophagitis and improvement of symptoms of gastroesophageal reflux disease after esomeprazole treatment in children 12 to 36 months old. Journal of Pediatric Gastroenterology and Nutrition 2010;51(5):593-8. - PubMed
Tolia 2010b {published data only (unpublished sought but not used)}
-
- Tolia V, Youssef NN, Gilger MA, Traxler B, Illueca M. Esomeprazole for the treatment of erosive esophagitis in children: an international, multicenter, randomized, parallel-group, double-blind (for dose) study. Journal of Pediatric Gastroenterology and Nutrition 2015;10(1):S24-30. - PubMed
Tsou 2006 {published data only}
-
- Tsou VM, Baker R, Book L, Hammo AH, Soffer EF, Wang W, et al. Multicenter, randomized, double-blind study comparing 20 and 40 mg of pantoprazole for symptom relief in adolescents (12 to 16 years of age) with gastroesophageal reflux disease (GERD). Clinical Pediatrics 2006;45(8):741-9. - PubMed
Ummarino 2015 {published data only}
-
- Ummarino D, Miele E, Martinelli M, Scarpato E, Crocetto F, Sciorio E, et al. Effect of magnesium alginate plus simethicone on gastroesophageal reflux in infants. Journal of Pediatric Gastroenterology and Nutrition 2015;60(2):230-5. - PubMed
Zohalinezhad 2015 {published data only}
-
- Zohalinezhad ME, Imanieh MH, Samani SM, Mohagheghzadeh A, Dehghani SM, Haghighat M, et al. Effects of quince syrup on clinical symptoms of children with symptomatic gastroesophageal reflux disease: a double-blind randomized controlled clinical trial. Complementary Therapies in Clinical Practice 2015;21(4):268-76. - PubMed
-
- Zohalinezhad ME, Zohalinezhad IS, Samani A, Mohagheghzadeh F, Dehghani H. Efficacy of quince (Cydonia oblonga M) syrup in symptomatic gastroesophageal reflux disease: a double-blind randomized controlled clinical trial. Planta Medica 2016;82(1):1439.
References to studies excluded from this review
Al‐Biltagi 2012 {published data only}
-
- Al-Biltagi M, Bediwy AS, Deraz S, Amer HG, Saeed NK. Esomeprazole, versus esomeprazole and domperidone in treatment of gastroesophageal reflux in children with difficult-to-treat asthma. European Respiratory Journal 2013;42(Suppl 57):1138. [NCT ID: NCT04821310]
Ameen 2006 {published data only}
-
- Ameen VZ, Pobiner BF, Giguere GC, Carter EG. Ranitidine (Zantac) syrup vs ranitidine effervescent tablets (Zantac EFFERdose) in children, a single-center taste preference study. Pediatric Drugs 2006;8(4):265-70. - PubMed
Bestebreurtje 2017 {published data only}
-
- Bestebreurtje P, De Koning B, Knibbe CA, Tibboel D, De Wildt SN. Rectal omeprazole in young infants: the magic bullet. Clinical Pharmacology and Therapeutics 2017;101(S1):S22.
Bestebreurtje 2020 {published data only}
Corvaglia 2010 {published data only}
-
- Corvaglia L, Aceti A, Mariani E, De Giorgi M, Capretti MG, Faldella G. The efficacy of sodium alginate (Gaviscon) for the treatment of gastro-oesophageal reflux in preterm infants. Alimentary Pharmacology and Therapeutics 2011;33:466-70. - PubMed
Dhillon 2004 {published data only}
-
- Dhillon AS, Ewer AK. Diagnosis and management of gastro-oesophageal reflux in preterm infants in neonatal intensive care units. Acta Paediatrica 2004;93:88-93. - PubMed
Fiedorek 2005 {published data only}
-
- Fiedorek S, Tolia V, Gold BD, Huang B, Stolle J, Lee C, et al. Efficacy and safety of lansoprazole in adolescents with symptomatic erosive and non-erosive gastro-oesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2005;40(3):319-27. - PubMed
Franco 2000 {published data only}
-
- Franco MT, Salvia G, Terrin G, Spadaro R, De Rosa I, Iula VD, et al. Lansoprazole in the treatment of gastro-oesophageal reflux disease in childhood. Digestive and Liver Disease 2000;32(8):660-6. - PubMed
Gunesekaran 1993 {published data only}
-
- Gunasekaran TS, Hassall EG. Efficacy and safety of omeprazole for severe gastroesophageal reflux in children. Journal of Pediatrics 1994;124(2):332-4. - PubMed
Haddad 2014 {published data only}
-
- Haddad I, Kierkus J, Tron E, Ulmer A, Hu P, Sloan S, et al. Maintenance of efficacy and safety of rabeprazole in children with endoscopically proven GERD. Journal of Pediatric Gastroenterology and Nutrition 2014;58(4):510-7. - PubMed
Hassall 2000 {published data only}
-
- Hassall E, Israel D, Shepherd R, Radke M, Dalväg A, Sköld B, et al. Omeprazole for treatment of chronic erosive esophagitis in children: a multicenter study of efficacy safety, tolerability and dose requirements. Journal of Pediatrics 2000;137(6):800-7. - PubMed
Hassall 2012 {published data only}
-
- Hassall E, Shepherd R, Koletzko S, Radke M, Henderson C, Lundborg P. Long-term maintenance treatment with omeprazole in children with healed erosive oesophagitis: a prospective study. Alimentary Pharmacology and Therapeutics 2012;35:368-79. - PubMed
James 2007 {published data only}
-
- James L, Walson P, Lomax K, Kao R, Varughese S, Reyes J. Pharmacokinetics and tolerability of rabeprazole sodium in subjects aged 12 to 16 years with gastroesophageal reflux disease: an open-label, single- and multiple-dose study. Clinical Therapeutics 2007;29(9):2082-92. - PubMed
Kaguelidou 2016 {published data only}
Kukulka 2012 {published data only}
-
- Kukulka M, Wu J, Perez MC. Pharmacokinetics and safety of dexlansoprazole MR in adolescents with symptomatic GERD. Journal of Pediatric Gastroenterology and Nutrition 2012;54(1):41-7. - PubMed
Kushki 2020 {unpublished data only}
-
- Kushki AM. Study of the effect of Esomeprazole and combination of Esomeprazole plus Baclofen in the treatment of clinical signs of infants reflux [sic]. Iranian Registry of Clinical Trials 2021.
Li 2006 {published data only}
-
- Li J, Zhao J, Hamer-Maansson JE, Andersson T, Fulmer R, Illueca M, et al. Pharmacokinetic properties of esomeprazole in children aged 1 to 11 years with symptoms of gastroesophageal reflux disease: a randomized, open-label study. Clinical Therapeutics 2006;28(11):1868-76. - PubMed
Loots 2011 {published data only}
-
- Loots CM, Smits MJ, Wijnakker R, Wijk MP, Davidson G, Benninga MA, et al. Esophageal impedance baselines in infants before and after placebo, antacid and proton pump inhibitor therapy. Journal of Pediatric Gastroenterology and Nutrition 2011;53(2):S68.
Madrazo‐de la Garza 2003 {published data only}
-
- Madrazo-de la Garza A, Dibildox M, Vargas A, Delgado J, Gonzalez J, Yañez P. Efficacy and safety of oral pantoprazole 20 mg given once daily for reflux esophagitis in children. Journal of Pediatric Gastroenterology and Nutrition 2003;36(2):261-5. - PubMed
Martin 2006 {published data only}
-
- Martin PB, Imong SM, Krischer J, Noblett HR, Sandhu BK. The use of omeprazole for resistant oesophagitis in children. European Journal of Pediatric Surgery 1996;6(4):195-7. - PubMed
Nielsen 2004 {published data only}
-
- Nielsen RG, Bindslev-Jensen C, Kruse-Andersen S, Husby S. Severe gastroesophageal reflux disease and cow milk hypersensitivity in infants and children: disease association and evaluation of a new challenge procedure. Journal of Pediatric Gastroenterology and Nutrition 2004;39(4):383-91. - PubMed
Omari 2009 {published data only}
-
- Omari T, Lundborg P, Sandstrom M, Bondarov P, Fjellman M, Haslam R, et al. Pharmacodynamics and systemic exposure of esomeprazole in preterm infants and term neonates with gastroesophageal reflux disease. Journal of Pediatrics 2009;155(2):222-8. - PubMed
Orenstein 2005 {published data only}
-
- Orenstein SR, Gremse DA, Pantaleon CD, Kling DF, Rotenberg KS. Nizatidine for the treatment of pediatric gastroesophageal reflux symptoms: an open-label, multiple-dose, randomized, multicenter clinical trial in 210 children. Clinical Therapeutics 2005;27(4):472-83. - PubMed
Orsi 2011 {published data only}
-
- Orsi M, Donato G, Busoni V, Naisberg G, Caruso N. Gastric acid suppression of a new oral powder omeprazole suspension for infants with gastroesophageal reflux disease. A pilot study [Eficacia acidosupresora del omeprazol en polvo en lactantes con reflujo gastroesofagico. Estudio piloto]. Acta Gastroenterologica Latinoamericana 2011;41(2):111-8. - PubMed
Pfizer 2021 {unpublished data only}
-
- Pfizer. Protonix (Pantoprazole) treatment of maintenance of healing in pediatric participants aged 1-11 years and 12-17 years. clinicaltrials.gov/ct2/show/NCT04821310 (first posted 29 March 2021).
Rabie 2016 {published and unpublished data}
-
- Rabie DE, Elshimi MS, Eltahery AM, Abusaif IS, Abushady NM, Ismail RI. G401 (P) Evaluation of gastroesophageal reflux disease in neonates pre and post proton pump inhibitors therapy using oesophageal multichannel intraluminal impedance and PH monitoring. Archives of Disease in Childhood 2016;101 (Suppl):A234-5.
Sabahi 2020 {unpublished data only}
-
- IRCT20191027045256N1. Effect of omeprazole and es-omeprazole in reflux disease [sic]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20191027045256N1 (first registered 09 March 2020).
Salvatore 2006 {published data only}
-
- Salvatore S, Hauser B, Salvatoni A, Vandenplas Y. Oral ranitidine and duration of gastric pH <4.0 in infants with persisting reflux symptoms. Acta Paediatrica 2006;95(2):176-81. - PubMed
Salvatore 2018 {published data only}
Størdal 2005 {published data only}
Tammara 2011 {published data only}
-
- Tammara BK, Sullivan JE, Adcock KG, Kierkus J, Giblin J, Rath N, et al. Randomized, open-label, multicentre pharmacokinetic studies of two dose levels of pantoprazole granules in infants and children aged 1 month through < 6 years with gastro-oesophageal reflux disease. Clinical Pharmacokinetics 2011;50(8):541-50. - PubMed
Terrin 2012 {published data only}
-
- Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics 2012;129:e40. - PubMed
Tolia 2002 {published data only}
-
- Tolia V, Ferry G, Gunesekaran T, Huang B, Keith R, Book L. Efficacy of lansoprazole in the treatment of gastrooesophageal reflux in children. Journal of Pediatric Gastroenterology and Nutrition 2002;35:S308-18. - PubMed
Tran 2002 {published data only}
-
- Tran A, Rey E, Pons G, Pariente-Khayat A, D'Athis P, Sallerin V, et al. Pharmacokinetic-pharmacodynamic study of oral lansoprazole in children. Clinical Pharmacology and Therapeutics 2002;71(5):359-67. - PubMed
Treepongkaruna 2011 {published data only}
-
- Treepongkaruna S, Ngoenmak T, Petchsrikul K, Aroonwan Preutthipan RN. Effect of lansoprazole plus domperidone for treatment of gastroesophageal reflux disease in infants. Journal of Pediatric Gastroenterology and Nutrition 2011;53(2):S69.
Ward 2011 {published data only}
-
- Ward RM, Kearns GL, Tammara B, Bishop P, O’Gorman MA, James LP, et al. A multicenter, randomized, open-label, pharmacokinetics and safety study of pantoprazole tablets in children and adolescents aged 6 through 16 years with gastroesophageal reflux disease. Journal of Clinical Pharmacology 2011;51(6):876-87. - PMC - PubMed
Winter 2010 {published data only}
-
- Winter H, Kum-Nji P, Mahomedy SH, Kierkus J, Hinz M, Li H, et al. Efficacy and safety of pantoprazole delayed-release granules for oral suspension in a placebo-controlled treatment-withdrawal study in infants 1-11 months old with symptomatic GERD. Journal of Pediatric Gastroenterology and Nutrition 2010;50(6):609-18. - PubMed
Winter 2012 {published data only}
-
- Winter H, Gunasekaran T, Tolia V, Gottrand F, Barker PN, Illueca M. Esomeprazole for the treatment of GERD in infants ages 1-11 months. Journal of Pediatric Gastroenterology and Nutrition 2012;55(1):14-20. - PubMed
-
- Winter H, Gunasekaran T, Tolia V, Gottrand F, Barker PN, Illueca M. Esomeprazole for the treatment of GERD in infants ages 1-11 months. Journal of Pediatric Gastroenterology and Nutrition 2015;60:S9-15. - PubMed
Zannikos 2011 {published data only}
-
- Zannikos PN, Doose DR, Leitz GJ, Rusch S, Gonzalez MD, Solanki B, et al. Pharmacokinetics and tolerability of rabeprazole in children 1 to 11 years old with gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2011;52(6):691-702. - PubMed
Zhao 2006 {published data only}
-
- Zhao J, Li J, Hamer-Maansson JE, Andersson T, Fulmer R, Illueca M, et al. Pharmacokinetic properties of esomeprazole in children aged 1 to 11 years with symptoms of gastroesophageal reflux disease: a randomized, open-label study. Clinical Therapeutics 2006;28(11):1868-76. - PubMed
References to studies awaiting assessment
Paknejad 2021 {published data only}
-
- Paknejad MS, Eftekhari K, Rahimi R, Vigeh M, Naghizadeh A, Karimi M. Myrtle (Myrtus communis L.) fruit syrup for gastroesophageal reflux disease in children: a double-blind randomized clinical trial. Phytotherapy Research 2021;35(11):6369-76. - PubMed
Shahmirzadi 2020 {published data only}
-
- Sobhani Shahmirzadi M, Barati L, Ebraimi M, Shiroodbakhshi K. The efficacy of baclofan to treat gastroesophageal reflux disease in children aged 6 months to 12 years: a clinical trial study. International Journal of Pediatrics 2020;8(5):11287-96.
Additional references
Augood 2003
Bashashati 2016
-
- Bashashati M, Sarosiek I, Siddiqui T, McCallum R. Adverse effects of domperidone: prolonged quest for knowledge? Digestive Diseases and Sciences 2016;61:3384-6. - PubMed
BNFc 2021
-
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, Neonatal and Paediatric Pharmacists Group. British National Formulary for Children. London, UK: BMJ Group, Pharmaceutical Press, RCPCH Publications, 2021.
Campanozzi 2009
-
- Campanozzi A, Boccia G, Pensabene L, Panetta F, Marseglia A, Strisciuglio P, et al. Prevalence and natural history of gastroesophageal reflux: pediatric prospective survey. Pediatrics 2009;123(3):779-83. - PubMed
Com Safety Med 2000
-
- Committee on Safety of Medicines and Medicines Control Agency. Cisapride (Propulsid) withdrawn. Curr Prob Pharmacovigil 2000;26:9-14.
Cooper 2002
-
- Cooper WO, Griffin MR, Arbogast P, et al. Very early exposure to erythromycin andinfantile hypertrophic pyloric stenosis. Archives of Pediatrics & Adolescent Medicine 2002;156:647-50. - PubMed
Craig 2004
De Bruyne 2018
-
- De Bruyne P, Ito S. Toxicity of long-term use of proton pump inhibitors in children. Archives of Disease in Childhood 2018;103(1):78-82. - PubMed
EMA 2014a
-
- European Medicine Agency Committee on Medicinal Products for Human Use. European Medicines Agency recommends changes to the use of metoclopramide. www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/07/... Accessed prior to 20 May 2023.
EMA 2014b
-
- European Medicines Agency. PRAC recommends restricting use of domperidone; March 2014. www.ema.europa.eu/en/news/prac-recommends-restricting-use-domperidone\ Accessed prior to 20 May 2023. [EMA: EMA/129231/2014]
FDA 2009
-
- Food and Drug Administration (FDA). FDA requires boxed warning and risk mitigation strategy for metoclopramide-containing drugs [press release]. Food and Drug Administration; Rockville, MD 2009.
Fitzgerald 2003
-
- Fitzgerald JF, Vasundhara T, Fraga P, Peters S, Donnelly C, Mack M, et al. Age-specific questionaires distinguish symptom frequency and severity in young children with and without gastroesophageal reflux disease. In: Sixteenth Annual Meeting of NASPGHAN Poster, Montreal, Quebec. 2003.
Fleishmann 2021
-
- Fleishman N, Richardson T, Attard T. The clinical characteristics of fractures in pediatric patients exposed to proton pump inhibitors. Journal of Pediatric Gastroenterology and Nutrition 2020;70(6):815-9. - PubMed
Franckx 1984
-
- Franckx J, Noel P. Acute extrapyramidal dysfunction after domperidone administration: Report of a case. Helvetica Paediatrica Acta 1984;39(3):285-8. - PubMed
Gaviscon Product Information 2021
-
- Reckitt Benckiser Healthcare (UK) Limited. Gaviscon Product Information. Reckitt Benckiser Healthcare (UK) Limited, Dansom Lane, Hull.
Hassall 2005
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hyams 1988
-
- Hyams JS, Ricci A Jr, Leichtner AM. Clinical and laboratory correlates of esophagitis. Journal of Pediatric Gastroenterology and Nutrition 1988;7(1):52-6. - PubMed
Hyams 2016
-
- Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2016;150:1456–68.
Hyman 1985
-
- Hyman PE, Abrams C, Garvey TO. Ranitidine tachyphylaxis. Gastroenterology 1985;88:1426.
Keady 2007
-
- Keady S. Update on drugs for gastro-oesophageal reflux disease. Archives of Disease in Childhood 2007;92:ep114-ep118. - PubMed
Kleinman 2011
-
- Kleinman L, Nelson S, Kothari-Talwar S, Roberts L, Orenstein SR, Mody RR, et al. Development and psychometric evaluation of 2 age-stratified versions of the Pediatric GERD Symptom and Quality of Life Questionnaire. Journal of Pediatric Gastroenterology and Nutrition 2011;52(5):514-22. - PubMed
Kwok 2017
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1-34. - PubMed
Mandel 2000
-
- Mandel KG, Daggy BP, Brodie DA, Jacoby HI. Review article: alginate-raft formulations in the treatment of heartburn and acid reflux. Alimentary Pharmacology Therapeutics 2000;14(6):669-90. - PubMed
Martin 2002
-
- Martin AJ, Pratt N, Kennedy JD, Ryan P, Ruffin RE, Miles H, et al. Natural history and familial relationships of infant spilling to 9 years of age. Pediatrics 2002;109(6):1061-7. - PubMed
McRorie 2014
MHRA 2012
-
- Medicines Health Regulatory Authority. Proton pump inhibitors in long-term use: reports of hypomagnesaemia. Drug Safety Update 2012;5(9):1.
MHRA 2019
-
- Medicines and Healthcare products Regulatory Agency. Ranitidine – MHRA drug alert issued for Teva UK recall, 2019. Available at: https://www.gov.uk/government/news/ ranitidine-mhra-drug-alert-issued-for-teva-uk-recall.
Miyazawa 2002
-
- Miyazawa R, Tomomasa T, Kaneko H, Tachibana A, Ogawa T, Morikawa A. Prevalence of gastro-esophageal reflux-related symptoms in Japanese infants. Pediatrics International 2002;44(5):513-6. - PubMed
NASPGHAN‐ESPGHAN guidelines 2018
-
- Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2018;66(3):516-54. [DOI: 10.1097/MPG.0b013e3181b7f563] - DOI - PMC - PubMed
Nelson 1997
-
- Nelson SP, Chen EH, Syniar GM, Christoffel K. Prevalence of symptoms of gastro-oesophageal reflux during infancy: a paediatric practice-based survey. Archives of Pediatrics and Adolescent Medicine 1997;151:569-72. - PubMed
NICE 2019
-
- National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease in children and young people: diagnosis and management (NG1); October 2019. www.nice.org.uk/guidance/ng1. - PubMed
Orenstein 2010
-
- Orenstein SR. Symptoms and reflux in infants: Infant Gastroesophageal Reflux Questionnaire Revised (I-GERQ-R): utility for symptom tracking and diagnosis. Curr Gastroenterol Rep. 2010;12(6):431–6. - PubMed
Page 2020
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
RevMan 2019 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2019.
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Schoenfeld 2016
-
- Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176(2):172-4. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013 Available from guidelinedevelopment.org/handbook. Available from guidelinedevelopment.org/handbook.
Shafrir 1985
-
- Shafrir Y, Levy Y, Ben-Amitai D, Nitzan M, Steinherz R. Oculogyric crisis due to domperidone therapy. Helvetica Paediatrica Acta 1985;40(1):95. - PubMed
Sherman 2009
-
- Sherman PM, Hassall E, Fagundes-Neto U, Gold BD, Kato S, Koletzko S, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. American Journal of Gastroenterology 2009;104(5):1278-95. - PubMed
Tighe 2009
-
- Tighe MP, Afzal NA, Bevan A, Beattie RM. Current pharmacological management of gastro-esophageal reflux in children. Pediatric Drugs 2009;11(3):185-202. - PubMed
Vandenplas 1999
Ward 2013
World Bank 2022
-
- World Bank. Country and Lending Groups classification of countries by income. datatopics.worldbank.org/world-development-indicators/the-world-by-incom... Accessed prior to 20 May 2023.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

