SynRoute: A Retrosynthetic Planning Software
- PMID: 37635298
- PMCID: PMC10498441
- DOI: 10.1021/acs.jcim.3c00491
SynRoute: A Retrosynthetic Planning Software
Abstract
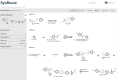
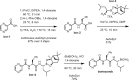
Computer-assisted synthetic planning has seen major advancements that stem from the availability of large reaction databases and artificial intelligence methodologies. SynRoute is a new retrosynthetic planning software tool that uses a relatively small number of general reaction templates, currently 263, along with a literature-based reaction database to find short, practical synthetic routes for target compounds. For each reaction template, a machine learning classifier is trained using data from the Pistachio reaction database to predict whether new computer-generated reactions based on the template are likely to work experimentally in the laboratory. This reaction generation methodology is used together with a vectorized Dijkstra-like search of top-scoring routes organized by synthetic strategies for easy browsing by a synthetic chemist. SynRoute was able to find routes for an average of 83% of compounds based on selection of random subsets of drug-like compounds from the ChEMBL database. Laboratory evaluation of 12 routes produced by SynRoute, to synthesize compounds not from the previous random subsets, demonstrated the ability to produce feasible overall synthetic strategies for all compounds evaluated.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Corey E. J.; Wipke W. T.; Cramer R. D.; Howe W. J. Computer-assisted synthetic analysis. Facile man-machine communication of chemical structure by interactive computer graphics. J. Am. Chem. Soc. 1972, 94, 421–430. 10.1021/ja00757a020. - DOI
-
- Corey E. J.; Cramer R. D.; Howe W. J. Computer-assisted synthetic analysis for complex molecules. Methods and procedures for machine generation of synthetic intermediates. J. Am. Chem. Soc. 1972, 94, 440–459. 10.1021/ja00757a022. - DOI
-
- Pensak D. A.; Corey E. J. LHASA—Logic and Heuristics Applied to Synthetic Analysis. Computer-Assisted Organic Synthesis; ACS Symposium Series; 1977; Vol. 61, Chapter 1, pp 1–32, 10.1021/bk-1977-0061.ch001. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

