Development of Enzymatic Recombinase Amplification Assays for the Rapid Visual Detection of HPV16/18
- PMID: 37635316
- PMCID: PMC10468672
- DOI: 10.4014/jmb.2304.04009
Development of Enzymatic Recombinase Amplification Assays for the Rapid Visual Detection of HPV16/18
Abstract
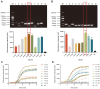
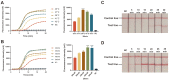
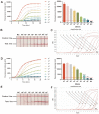
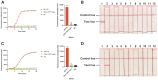
Human papillomavirus (HPV) types 16 and 18 are the major causes of cervical lesions and are associated with 71% of cervical cancer cases globally. However, public health infrastructures to support cervical cancer screening may be unavailable to women in low-resource areas. Therefore, sensitive, convenient, and cost-efficient diagnostic methods are required for the detection of HPV16/18. Here, we designed two novel methods, real-time ERA and ERA-LFD, based on enzymatic recombinase amplification (ERA) for quick point-of-care identification of the HPV E6/E7 genes. The entire detection process could be completed within 25 min at a constant low temperature (35-43°C), and the results of the combined methods could be present as the amplification curves or the bands presented on dipsticks and directly interpreted with the naked eye. The ERA assays evaluated using standard plasmids carrying the E6/E7 genes and clinical samples exhibited excellent specificity, as no cross-reaction with other common HPV types was observed. The detection limits of our ERA assays were 100 and 101 copies/μl for HPV16 and 18 respectively, which were comparable to those of the real-time PCR assay. Assessment of the clinical performance of the ERA assays using 114 cervical tissue samples demonstrated that they are highly consistent with real-time PCR, the gold standard for HPV detection. This study demonstrated that ERA-based assays possess excellent sensitivity, specificity, and repeatability for HPV16 and HPV18 detection with great potential to become robust diagnostic tools in local hospitals and field studies.
Keywords: Enzymatic recombinase amplification; human papillomavirus; lateral flow dipstick; rapid visual detection.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures