The Role of Calcium Hydroxylapatite (Radiesse) as a Regenerative Aesthetic Treatment: A Narrative Review
- PMID: 37635437
- PMCID: PMC11025388
- DOI: 10.1093/asj/sjad173
The Role of Calcium Hydroxylapatite (Radiesse) as a Regenerative Aesthetic Treatment: A Narrative Review
Abstract
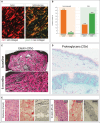
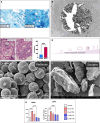
For decades, a wide variety of natural and synthetic materials have been used to augment human tissue to improve aesthetic outcomes. Dermal fillers are some of the most widely used aesthetic treatments throughout the body. Initially, the primary function of dermal fillers was to restore depleted volume. As biomaterial research has advanced, however, a variety of biostimulatory fillers have become staples in aesthetic medicine. Such fillers often contain a carrying vehicle and a biostimulatory material that induces de novo synthesis of major structural components of the extracellular matrix. One such filler, Radiesse (Merz Aesthetics, Raleigh, NC), is composed of calcium hydroxylapatite microspheres suspended in a carboxymethylcellulose gel. In addition to immediate volumization, Radiesse treatment results in increases of collagen, elastin, vasculature, proteoglycans, and fibroblast populations via a cell-biomaterial-mediated interaction. When injected, Radiesse acts as a cell scaffold and clinically manifests as immediate restoration of depleted volume, improvements in skin quality and appearance, and regeneration of endogenous extracellular matrices. This narrative review contextualizes Radiesse as a regenerative aesthetic treatment, summarizes its unique use cases, reviews its rheological, material, and regenerative properties, and hypothesizes future combination treatments in the age of regenerative aesthetics.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Aesthetic Society.
Figures










References
-
- Mehta H, Bishnoi A, Dogra S. Regenerative medicine in aesthetics. CosmoDerma. 2022;2(41):1-7. doi: 10.25259/CSDM_46_2022 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

