Green Synthesized Silver Nanoparticles: A Potential Antibacterial Agent, Antioxidant, and Colorimetric Nanoprobe for the Detection of Hg2+ Ions
- PMID: 37635703
- PMCID: PMC10448124
- DOI: 10.1002/gch2.202300072
Green Synthesized Silver Nanoparticles: A Potential Antibacterial Agent, Antioxidant, and Colorimetric Nanoprobe for the Detection of Hg2+ Ions
Abstract
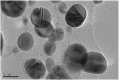
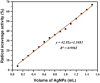
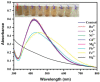
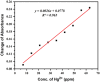
Silver nanoparticles (AgNPs) prepared by green synthesis have a lot of potentials in various fields. Among them, as an antioxidant, antibacterial agent, and nanoprobe for the colorimetric detection of mercury (Hg2+) ions is thought to be the most important. The antibacterial, antioxidant, and colorimetric sensing potential of the greenly produced AgNPs utilizing Piper chaba stem extract are all predicted in this investigation. By using the disc diffusion method, the antibacterial activity of greenly produced AgNPs are assessed, and the findings are measured from the zone of inhibition (ZOI). It is revealed that the Staphylococcus aureus, Micrococcus spp., Escherichia coli, and Pseudomonas aeruginosa bacterial strains are significantly resisted by the greenly produced AgNPs. The antioxidant activity test of AgNPs reveals a considerable impact on free radical scavenging having the inhibitory concentration (IC 50) is 1.13 mL (equivalent to 0.45 mg mL-1). Also, with a low limit of detection of 28 ppm, the resulting AgNPs are used as highly selective and economical colorimetric sensors for Hg2+ detection. The study's findings support the hypothesis that Piper chaba stems can serve as a source for the production of AgNPs with high antibacterial and antioxidant activity and usefulness for simple colorimetric readings of Hg2+.
Keywords: antibacterial agent; antioxidant; mercury detection; nanoprobe; silver nanoparticles.
© 2023 The Authors. Global Challenges published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Green synthesis of Piper chaudocanum stem extract mediated silver nanoparticles for colorimetric detection of Hg2+ ions and antibacterial activity.R Soc Open Sci. 2023 Feb 8;10(2):220819. doi: 10.1098/rsos.220819. eCollection 2023 Feb. R Soc Open Sci. 2023. PMID: 36778963 Free PMC article.
-
Synthesis of hydroxyethylcellulose phthalate-modified silver nanoparticles and their multifunctional applications as an efficient antibacterial, photocatalytic and mercury-selective sensing agent.Int J Biol Macromol. 2024 Jan;256(Pt 1):128009. doi: 10.1016/j.ijbiomac.2023.128009. Epub 2023 Nov 22. Int J Biol Macromol. 2024. PMID: 37995781
-
Colorimetric detection of Hg(II) by γ-aminobutyric acid-silver nanoparticles in water and the assessment of antibacterial activities.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Apr 15;251:119433. doi: 10.1016/j.saa.2021.119433. Epub 2021 Jan 6. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33465574
-
Causonis trifolia-based green synthesis of multifunctional silver nanoparticles for dual sensing of mercury and ferric ions, photocatalysis, and biomedical applications.RSC Adv. 2025 May 20;15(21):16879-16893. doi: 10.1039/d5ra01882j. eCollection 2025 May 15. RSC Adv. 2025. PMID: 40395792 Free PMC article.
-
Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles From Azadirachta indica.Front Microbiol. 2021 Feb 19;12:611560. doi: 10.3389/fmicb.2021.611560. eCollection 2021. Front Microbiol. 2021. PMID: 33679635 Free PMC article.
Cited by
-
Green synthesis of silver nanoparticles and its environmental sensor ability to some heavy metals.BMC Chem. 2024 Jan 6;18(1):7. doi: 10.1186/s13065-023-01105-y. BMC Chem. 2024. PMID: 38184656 Free PMC article.
-
Waste upcycling of Sapota peels as a green route for the synthesis of silver nanoparticles and their application as catalytic and colorimetric detection of Co2+ and Hg2.Discov Nano. 2024 Nov 21;19(1):191. doi: 10.1186/s11671-024-04147-w. Discov Nano. 2024. PMID: 39572462 Free PMC article.
References
-
- Adhikari A., Lamichhane L., Adhikari A., Gyawali G., Acharya D., Baral E. R., Chhetri K., Inorganics 2022, 10, 113.
-
- Panáček A., Kvítek L., Prucek R., Kolář M., Večeřová R., Pizúrová N., Sharma V. K., Nevěčná T., Zbořil R., J. Phys. Chem. B 2006, 110, 16248. - PubMed
-
- McFarland A. D., Van Duyne R. P., Nano Lett. 2003, 3, 1057.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

