Octanoic acid promotes clearance of antibiotic-tolerant cells and eradicates biofilms of Staphylococcus aureus isolated from recurrent bovine mastitis
- PMID: 37635811
- PMCID: PMC10450856
- DOI: 10.1016/j.bioflm.2023.100149
Octanoic acid promotes clearance of antibiotic-tolerant cells and eradicates biofilms of Staphylococcus aureus isolated from recurrent bovine mastitis
Abstract
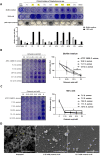
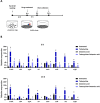
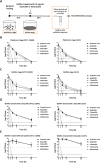
Antibiotic therapy is the primary treatment for bovine mastitis, but the drawbacks of this strategy include poor cure rate and economic losses from the need to discard milk with antibiotic residues. Unfortunately, few other treatment options are currently available for mastitis. Failure of antibiotic treatments is often attributed to formation of bacterial biofilms and abscesses in the mammary gland tissue, which lead to chronic infections that are difficult to eradicate and drive recurrent disease. A major mastitis-causing pathogen (MCP) associated with biofilms in bovine mastitis is Staphylococcus aureus. In this study, we demonstrate that octanoic acid has broad-spectrum microbicidal activity against MCPs and effectively inhibits S. aureus biofilm formation in milk (>50% inhibition at 3.13 mM). Octanoic acid effectively clears biofilms (95% eradication at 1X minimum bactericidal concentration, MBC) and infrequently induces S. aureus small colony variants (SCVs) that may cause recurrent mastitis. Additionally, octanoic acid rapidly kills persistent biofilm cells and cells with antibiotic tolerance (within 4 h). In contrast, antibiotics treated at >100X MBC cannot eradicate biofilms but do induce SCVs and antibiotic-tolerant cells. These effects may accelerate the transition from biofilm to chronic infection. Thus, octanoic acid exhibits bactericidal action against S. aureus biofilms, and it is less likely than antibiotic therapy to induce persistent cells and pathogen tolerance. Moreover, octanoic acid acts additively with antibiotics against S. aureus, and it attenuates tetracycline-induced virulence factor gene expression in S. aureus cells. According to these data, octanoic acid may prevent the pathological progression of bovine mastitis and offer a new strategy for treating the condition.
Keywords: Antibiotic tolerance cells; Octanoic acid; Persistence cells; Recurrent mastitis; Staphylococcus aureus biofilms.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Mammary Gland Pathology Subsequent to Acute Infection with Strong versus Weak Biofilm Forming Staphylococcus aureus Bovine Mastitis Isolates: A Pilot Study Using Non-Invasive Mouse Mastitis Model.PLoS One. 2017 Jan 27;12(1):e0170668. doi: 10.1371/journal.pone.0170668. eCollection 2017. PLoS One. 2017. PMID: 28129375 Free PMC article.
-
Knema retusa is antibacterial and antibiofilm against antibiotic resistant Staphylococcus aureus and S. haemolyticus isolated in bovine mastitis.Vet Res Commun. 2023 Jun;47(2):523-538. doi: 10.1007/s11259-022-09999-0. Epub 2022 Oct 19. Vet Res Commun. 2023. PMID: 36260188
-
Prevalence, Antimicrobial Resistance, and Characterization of Staphylococcus aureus Isolated from Subclinical Bovine Mastitis in East Coast Malaysia.Animals (Basel). 2022 Jun 29;12(13):1680. doi: 10.3390/ani12131680. Animals (Basel). 2022. PMID: 35804578 Free PMC article.
-
Anti-virulence compounds against Staphylococcus aureus associated with bovine mastitis: A new therapeutic option?Microbiol Res. 2023 Jun;271:127345. doi: 10.1016/j.micres.2023.127345. Epub 2023 Mar 1. Microbiol Res. 2023. PMID: 36889204 Review.
-
Staphylococcus aureus persistence properties associated with bovine mastitis and alternative therapeutic modalities.J Appl Microbiol. 2020 Nov;129(5):1102-1119. doi: 10.1111/jam.14706. Epub 2020 Jun 8. J Appl Microbiol. 2020. PMID: 32416020 Review.
Cited by
-
Sex- and Metamorphosis-Related Changes in the Cuticular Lipid Profile of Galleria mellonella Pupae and Adults.Insects. 2024 Dec 4;15(12):965. doi: 10.3390/insects15120965. Insects. 2024. PMID: 39769567 Free PMC article.
-
The mechanistic role of natural antimicrobials in preventing Staphylococcus aureus invasion of MAC-T cells using an in vitro mastitis model.Ir Vet J. 2024 Feb 27;77(1):3. doi: 10.1186/s13620-024-00265-0. Ir Vet J. 2024. PMID: 38414081 Free PMC article.
-
Pharmacokinetics and relative bioavailability study of two cefquinome sulfate intramammary infusions in cow milk.Front Vet Sci. 2024 Mar 11;11:1384076. doi: 10.3389/fvets.2024.1384076. eCollection 2024. Front Vet Sci. 2024. PMID: 38528872 Free PMC article.
-
Anti-staphylococcal fatty acids: mode of action, bacterial resistance and implications for therapeutic application.Microbiology (Reading). 2025 May;171(5):001563. doi: 10.1099/mic.0.001563. Microbiology (Reading). 2025. PMID: 40402078 Free PMC article. Review.
-
Chemical Analysis and Antioxidant Activities of Resin Fractions from Pistacia lentiscus L. var. Chia in Neuroblastoma SH-SY5Y Cells.Molecules. 2025 Feb 21;30(5):997. doi: 10.3390/molecules30050997. Molecules. 2025. PMID: 40076222 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

