Age-dependent structural reorganization of utricular ribbon synapses
- PMID: 37635868
- PMCID: PMC10447907
- DOI: 10.3389/fcell.2023.1178992
Age-dependent structural reorganization of utricular ribbon synapses
Abstract
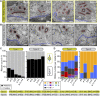
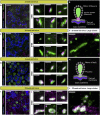
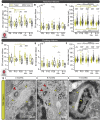
In mammals, spatial orientation is synaptically-encoded by sensory hair cells of the vestibular labyrinth. Vestibular hair cells (VHCs) harbor synaptic ribbons at their presynaptic active zones (AZs), which play a critical role in molecular scaffolding and facilitate synaptic release and vesicular replenishment. With advancing age, the prevalence of vestibular deficits increases; yet, the underlying mechanisms are not well understood and the possible accompanying morphological changes in the VHC synapses have not yet been systematically examined. We investigated the effects of maturation and aging on the ultrastructure of the ribbon-type AZs in murine utricles using various electron microscopic techniques and combined them with confocal and super-resolution light microscopy as well as metabolic imaging up to 1 year of age. In older animals, we detected predominantly in type I VHCs the formation of floating ribbon clusters, mostly consisting of newly synthesized ribbon material. Our findings suggest that VHC ribbon-type AZs undergo dramatic structural alterations upon aging.
Keywords: aging; ribbon synapse; synaptogenesis; utricle; vestibular hair cells.
Copyright © 2023 Michanski, Henneck, Mukhopadhyay, Steyer, Gonzalez, Grewe, Ilgen, Gültas, Fornasiero, Jakobs, Möbius, Vogl, Pangršič, Rizzoli and Wichmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Exocytotic machineries of vestibular type I and cochlear ribbon synapses display similar intrinsic otoferlin-dependent Ca2+ sensitivity but a different coupling to Ca2+ channels.J Neurosci. 2014 Aug 13;34(33):10853-69. doi: 10.1523/JNEUROSCI.0947-14.2014. J Neurosci. 2014. PMID: 25122888 Free PMC article.
-
Spaceflight-induced synaptic modifications within hair cells of the mammalian utricle.J Neurophysiol. 2017 Jun 1;117(6):2163-2178. doi: 10.1152/jn.00240.2016. Epub 2017 Feb 22. J Neurophysiol. 2017. PMID: 28228581 Free PMC article.
-
Functional and Structural Development of Mouse Cone Photoreceptor Ribbon Synapses.Invest Ophthalmol Vis Sci. 2022 Mar 2;63(3):21. doi: 10.1167/iovs.63.3.21. Invest Ophthalmol Vis Sci. 2022. PMID: 35319739 Free PMC article.
-
Balancing presynaptic release and endocytic membrane retrieval at hair cell ribbon synapses.FEBS Lett. 2018 Nov;592(21):3633-3650. doi: 10.1002/1873-3468.13258. Epub 2018 Oct 9. FEBS Lett. 2018. PMID: 30251250 Review.
-
Presynaptic [Ca(2+)] and GCAPs: aspects on the structure and function of photoreceptor ribbon synapses.Front Mol Neurosci. 2014 Feb 6;7:3. doi: 10.3389/fnmol.2014.00003. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24567702 Free PMC article. Review.
Cited by
-
A novel membrane contact site in vestibular hair cells.bioRxiv [Preprint]. 2025 Mar 24:2025.03.21.644540. doi: 10.1101/2025.03.21.644540. bioRxiv. 2025. PMID: 40196657 Free PMC article. Preprint.
-
KV1.8 (Kcna10) potassium channels enhance fast, linear signaling in vestibular hair cells and facilitate vestibulomotor reflexes and balance.bioRxiv [Preprint]. 2025 Jan 28:2025.01.28.634388. doi: 10.1101/2025.01.28.634388. bioRxiv. 2025. PMID: 39975259 Free PMC article. Preprint.
-
Morphological correlates of synaptic protein turnover in the mouse brain.Life Sci Alliance. 2024 Aug 12;7(11):e202402793. doi: 10.26508/lsa.202402793. Print 2024 Nov. Life Sci Alliance. 2024. PMID: 39134363 Free PMC article.
-
A Regional Ultrastructural Analysis of the Cellular and Synaptic Architecture of the Mouse Vestibular Periphery, With Reference to the Chinchilla.J Comp Neurol. 2025 Jul;533(7):e70074. doi: 10.1002/cne.70074. J Comp Neurol. 2025. PMID: 40702675 Free PMC article.
References
-
- Bertie A. (2002). Java applications for teaching statistics. MSOR Connect. 2, 78–81. 10.11120/msor.2002.02030078 - DOI
LinkOut - more resources
Full Text Sources

