A novel SATB1 protein isoform with different biophysical properties
- PMID: 37635874
- PMCID: PMC10457122
- DOI: 10.3389/fcell.2023.1242481
A novel SATB1 protein isoform with different biophysical properties
Abstract
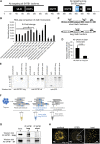
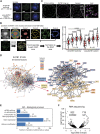
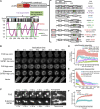
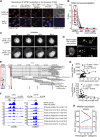
Intra-thymic T cell development is coordinated by the regulatory actions of SATB1 genome organizer. In this report, we show that SATB1 is involved in the regulation of transcription and splicing, both of which displayed deregulation in Satb1 knockout murine thymocytes. More importantly, we characterized a novel SATB1 protein isoform and described its distinct biophysical behavior, implicating potential functional differences compared to the commonly studied isoform. SATB1 utilized its prion-like domains to transition through liquid-like states to aggregated structures. This behavior was dependent on protein concentration as well as phosphorylation and interaction with nuclear RNA. Notably, the long SATB1 isoform was more prone to aggregate following phase separation. Thus, the tight regulation of SATB1 isoforms expression levels alongside with protein post-translational modifications, are imperative for SATB1's mode of action in T cell development. Our data indicate that deregulation of these processes may also be linked to disorders such as cancer.
Keywords: SATB1; T cells; chromatin organization; phase separation; prion.
Copyright © 2023 Zelenka, Papamatheakis, Tzerpos, Panagopoulos, Tsolis, Papadakis, Mariatos Metaxas, Papadogkonas, Mores, Kapsetaki, Papamatheakis, Stanek and Spilianakis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

