Cellular heterogeneity and plasticity during NAFLD progression
- PMID: 37635938
- PMCID: PMC10450943
- DOI: 10.3389/fmolb.2023.1221669
Cellular heterogeneity and plasticity during NAFLD progression
Erratum in
-
Corrigendum: Cellular heterogeneity and plasticity during NAFLD progression.Front Mol Biosci. 2024 Oct 28;11:1511947. doi: 10.3389/fmolb.2024.1511947. eCollection 2024. Front Mol Biosci. 2024. PMID: 39529685 Free PMC article.
Abstract
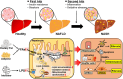
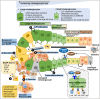
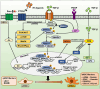
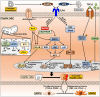
Nonalcoholic fatty liver disease (NAFLD) is a progressive liver disease that can progress to nonalcoholic steatohepatitis (NASH), NASH-related cirrhosis, and hepatocellular carcinoma (HCC). NAFLD ranges from simple steatosis (or nonalcoholic fatty liver [NAFL]) to NASH as a progressive form of NAFL, which is characterized by steatosis, lobular inflammation, and hepatocellular ballooning with or without fibrosis. Because of the complex pathophysiological mechanism and the heterogeneity of NAFLD, including its wide spectrum of clinical and histological characteristics, no specific therapeutic drugs have been approved for NAFLD. The heterogeneity of NAFLD is closely associated with cellular plasticity, which describes the ability of cells to acquire new identities or change their phenotypes in response to environmental stimuli. The liver consists of parenchymal cells including hepatocytes and cholangiocytes and nonparenchymal cells including Kupffer cells, hepatic stellate cells, and endothelial cells, all of which have specialized functions. This heterogeneous cell population has cellular plasticity to adapt to environmental changes. During NAFLD progression, these cells can exert diverse and complex responses at multiple levels following exposure to a variety of stimuli, including fatty acids, inflammation, and oxidative stress. Therefore, this review provides insights into NAFLD heterogeneity by addressing the cellular plasticity and metabolic adaptation of hepatocytes, cholangiocytes, hepatic stellate cells, and Kupffer cells during NAFLD progression.
Keywords: Cholangiocytes; Kupffer cells; NAFLD; cellular plasticity; hepatic stellate cells; hepatocytes; heterogeneity; metabolic adaptation.
Copyright © 2023 Park, Choi, Kim, Yang, An, Lee, Han, Lee, Kim, Bae and Oh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as potential conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

