Mometasone Furoate Use for Recurrent Adenoid Hypertrophy: Randomized Controlled Clinical Trial
- PMID: 37636767
- PMCID: PMC10447813
- DOI: 10.1007/s12070-023-03539-1
Mometasone Furoate Use for Recurrent Adenoid Hypertrophy: Randomized Controlled Clinical Trial
Abstract
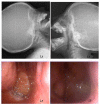
Background& Objective: Adenoid hypertrophy (AH) in children is one of the most causes of nasal obstruction and is associated with many nasal and respiratory symptoms. Till now, surgery is the main option for managing the associated symptom of AH. The intranasal steroid has an effective role in the control of allergic rhinitis and associated AH. This work aimed to assess the effects of local mometasone on recurrent AH in children. Patients& Methods: A randomized controlled trial enrolled 39 patients aged between 2 and 15 years with recurrent AH. Those patients were randomly subdivided into three groups; group (A) received topical mometasone furoate (MF), group (B) did not receive any medication, and group (C) received topical normal saline. All groups were followed up for 8 weeks. Results: Patients who received Mometasone furoate had temporary relief of adenoid hypertrophy-related symptoms (84.6%) in comparison to the control group and placebo group during the duration of treatment. After cessation of treatment with local steroids, all cases experienced symptoms caused by adenoid hypertrophy, and by the end of the third month of follow up all cases underwent adenoidectomy. Conclusion: Mometasone furoate can temporarily reduce the adenoid size, reducing symptoms related to adenoid hypertrophy.
Keywords: Adenoid hypertrophy; Hypo-nasal speech; Mometasone furoate; Naso-endoscopy.
© Association of Otolaryngologists of India 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing InterestsThe authors have no conflict of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
