Novel 2-Acetamido-2-ylidene-4-imidazole Derivatives (El-Saghier Reaction): Green Synthesis, Biological Assessment, and Molecular Docking
- PMID: 37636903
- PMCID: PMC10448697
- DOI: 10.1021/acsomega.3c03767
Novel 2-Acetamido-2-ylidene-4-imidazole Derivatives (El-Saghier Reaction): Green Synthesis, Biological Assessment, and Molecular Docking
Abstract
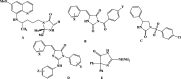
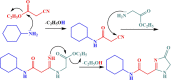
El-Saghier reaction is the novel, general, and green reaction of various amines with ethyl cyanoacetate and ethyl glycinate hydrochloride. A new series of imidazolidin-4-ones and bis-N-(alkyl/aryl) imidazolidin-4-ones was synthesized in a sequential, one-pot procedure under neat conditions for 2 h at 70 °C. Excellent high yields (90-98%) were achieved in a short period of time while avoiding issues related to the hazardous solvents utilized (cost, safety, and pollution). The spectrum analyses and elemental data of the newly synthesized compounds helped us to clarify their structures. The obtained compounds were tested for antibacterial activity in vitro and compared to the standard antibiotic chloramphenicol as the standard, measuring the inhibition zone (nm) and activity index (%). With an antibacterial percentage value of 80.0 against Escherichia coli, N,N'-(propane-1,3-diyl) bis(2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl) acetamide) proved to be the most effective. Antimicrobial activity was confirmed by a molecular docking investigation to investigate how chemicals bind to the bacterial FabH-CoA complex in E. coli (PDB ID: 1HNJ).
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Synthesis, antimicrobial activities, docking studies and computational calculations of new bis-1,4-phenylene -1H-1,2,3-triazole derivatives utilized ultrasonic energy.J Biomol Struct Dyn. 2022 Aug;40(12):5409-5426. doi: 10.1080/07391102.2021.1875051. Epub 2021 Feb 1. J Biomol Struct Dyn. 2022. PMID: 33522432
-
Superparamagnetic Poly(aniline-co-m-phenylenediamine)@Fe3O4 Nanocomposite as an Efficient Heterogeneous Catalyst for the Synthesis of 1H-pyrazolo[1,2-a]pyridazine-5,8-diones & 1H-pyrazolo[1,2-b]phthalazine-5, 10-diones Derivatives.Curr Org Synth. 2022 Mar 3;19(2):246-266. doi: 10.2174/1570179418666211104143736. Curr Org Synth. 2022. PMID: 34736384
-
Novel derivatives of methyl and ethyl 2-(4-oxo-8-aryl-2H-3,4,6,7-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)acetates from biologically active 1-aryl-2-hydrazinoimidazolines: synthesis, crystal structure and antiproliferative activity.Eur J Med Chem. 2006 Dec;41(12):1373-84. doi: 10.1016/j.ejmech.2006.06.013. Epub 2006 Sep 22. Eur J Med Chem. 2006. PMID: 16996655
-
Design, Synthesis and Molecular Docking of 1-Cyclopropyl-6- Fluoro-4-Oxo-7-{4-[2-(4-Substituted-Phenyl)-2-(Substituted)-Ethyl] -1-Piperazinyl}-1,4-Dihydroquinoline-3-Carboxylic Acid as an Antimicrobial Agents.Curr Drug Discov Technol. 2017;14(4):255-269. doi: 10.2174/1570163814666170224110500. Curr Drug Discov Technol. 2017. PMID: 28240185
-
Design, green one-pot synthesis and molecular docking study of novel N,N-bis(cyanoacetyl)hydrazines and bis-coumarins as effective inhibitors of DNA gyrase and topoisomerase IV.Bioorg Chem. 2020 Apr;97:103672. doi: 10.1016/j.bioorg.2020.103672. Epub 2020 Feb 16. Bioorg Chem. 2020. PMID: 32145481
Cited by
-
Exploring corrosion behavior, antimicrobial evaluation, molecular docking and DFT calculation of thiosemicarbazone ligand and its metal complexes.Sci Rep. 2025 May 13;15(1):16577. doi: 10.1038/s41598-025-98580-1. Sci Rep. 2025. PMID: 40360666 Free PMC article.
-
Synthesis, docking and biological evaluation of purine-5-N-isosteresas anti-inflammatory agents.RSC Adv. 2024 Jun 3;14(25):17785-17800. doi: 10.1039/d4ra02970d. eCollection 2024 May 28. RSC Adv. 2024. PMID: 38832248 Free PMC article.
-
Structural and topological analysis of thiosemicarbazone-based metal complexes: computational and experimental study of bacterial biofilm inhibition and antioxidant activity.BMC Chem. 2025 Jan 24;19(1):24. doi: 10.1186/s13065-024-01338-5. BMC Chem. 2025. PMID: 39856776 Free PMC article.
-
An efficient eco-friendly, simple, and green synthesis of some new spiro-N-(4-sulfamoyl-phenyl)-1,3,4-thiadiazole-2-carboxamide derivatives as potential inhibitors of SARS-CoV-2 proteases: drug-likeness, pharmacophore, molecular docking, and DFT exploration.Mol Divers. 2024 Feb;28(1):249-270. doi: 10.1007/s11030-023-10761-0. Epub 2023 Nov 9. Mol Divers. 2024. PMID: 37946070 Free PMC article.
-
Synthesis, docking and characterization of some novel 5-(S-alkyl)-1.3.4-thiadiazole-2-carboxamide derivatives as anti-inflammatory and antibacterial agents.BMC Chem. 2024 Jul 27;18(1):138. doi: 10.1186/s13065-024-01237-9. BMC Chem. 2024. PMID: 39068479 Free PMC article.
References
-
- Albayati M. R.; Kansız S.; Dege N.; Kaya S.; Marzouki R.; Lgaz H.; Salghi R.; Ali I. H.; Alghamdi M. M.; Chung I.-M. Synthesis, crystal structure, Hirshfeld surface analysis and DFT calculations of 2-[(2, 3-dimethylphenyl) amino]-N’-[(E)-thiophen-2-ylmethylidene] benzohydrazide. J. Mol. Struct. 2020, 1205, 127654.10.1016/j.molstruc.2019.127654. - DOI
-
- Mlostoń G.; Celeda M.; Jasiński M.; Urbaniak K.; Boratyński P. J.; Schreiner P. R.; Heimgartner H. 2-Unsubstituted imidazole N-oxides as novel precursors of chiral 3-alkoxyimidazol-2-ylidenes derived from trans-1, 2-diaminocyclohexane and other chiral amino compounds. Molecules 2019, 24, 4398.10.3390/molecules24234398. - DOI - PMC - PubMed
-
- Handzlik J.; Szymańska E.; Nędza K.; Kubacka M.; Siwek A.; Mogilski S.; Handzlik J.; Filipek B.; Kieć-Kononowicz K. Pharmacophore models based studies on the affinity and selectivity toward 5-HT1A with reference to α1-adrenergic receptors among arylpiperazine derivatives of phenytoin. Bioorg. Med. Chem. 2011, 19, 1349–1360. 10.1016/j.bmc.2010.11.051. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

