A New Saponin (Zygo-albuside D) from Zygophyllum album Roots Triggers Apoptosis in Non-Small Cell Lung Carcinoma (A549 Cells) through CDK-2 Inhibition
- PMID: 37636931
- PMCID: PMC10448641
- DOI: 10.1021/acsomega.3c04314
A New Saponin (Zygo-albuside D) from Zygophyllum album Roots Triggers Apoptosis in Non-Small Cell Lung Carcinoma (A549 Cells) through CDK-2 Inhibition
Abstract
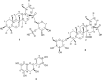
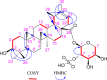
Phytochemical study of the ethyl acetate root extract of Zygophyllum album has resulted in the isolation of a new saponin, Zygo-albuside D (1), along with two known compounds; (3-O-[β-D-quinovopyranosyl]-quinovic acid) (2), which is first reported in the root, and catechin (3), first reported in the genus. Their chemical structures were established by NMR and high-resolution mass spectrometry (HRMS). The new saponin (1) exhibited promising cytotoxicity with IC50 values of 3.5 and 5.52 μM on A549 and PC-3 cancer cell lines, respectively, compared to doxorubicin with IC50 values of 9.44 and 11.39 μM on A549 and PC-3 cancer cell lines, respectively. While it had an IC50 value of 46.8 μM against WISH cells. Investigating apoptosis-induction, compound 1 induced total apoptotic cell death in A549 lung cancer cells by 32-fold; 21.53% compared to 0.67% in the untreated control cells. Finally, it upregulated the pro-apoptotic genes and downregulated the antiapoptotic gene using gene expression levels. Compound 1 exhibited remarkable CDK-2 target inhibition by 96.2% with an IC50 value of 117.6 nM compared to Roscovitine. The molecular docking study further confirmed the binding affinity of compound 1 as CDK2 and Bcl2 inhibitors that led to apoptosis induction in A549 cancer cells. Hence, this study highlights the importance of compound 1 in the design of a new anticancer agent with specific mechanisms.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Eguchi H.; Kimura R.; Matsunaga H.; Matsunaga T.; Yoshino Y.; Endo S.; Ikari A. Increase in Anticancer Drug-Induced Toxicity by Fisetin in Lung Adenocarcinoma A549 Spheroid Cells Mediated by the Reduction of Claudin-2 Expression. Int. J. Mol. Sci. 2022, 23, 7536.10.3390/ijms23147536. - DOI - PMC - PubMed
-
- Jain S.; Dwivedi J.; Jain P. K.; Satpathy S.; Patra A. Medicinal plants for treatment of cancer: A brief review. Pharmacogn J. 2016, 8, 87.10.5530/pj.2016.2.1. - DOI
-
- Abdelhameed R. F.; Habib E. S.; Ibrahim A. K.; Yamada K.; Abdel-Kader M. S.; Ahmed S. A.; Ibrahim A. K.; Badr J. M.; Nafie M. S. Chemical constituent profiling of Phyllostachys heterocycla var. Pubescens with selective cytotoxic polar fraction through EGFR inhibition in HepG2 cells. Molecules 2021, 26, 940.10.3390/molecules26040940. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

