Characterization of engraftment dynamics in myelofibrosis after allogeneic hematopoietic cell transplantation including novel conditioning schemes
- PMID: 37637037
- PMCID: PMC10449533
- DOI: 10.3389/fonc.2023.1205387
Characterization of engraftment dynamics in myelofibrosis after allogeneic hematopoietic cell transplantation including novel conditioning schemes
Abstract
Introduction: Myelofibrosis (MF) is a rare hematopoietic stem cell disorder progressing to bone marrow (BM) failure or blast phase. Allogeneic hematopoietic cell transplantation (HCT) represents a potentially curative therapy for a limited subset of patients with advanced MF, who are eligible, but engraftment in MF vs. AML is delayed which promotes complications. As determinants of engraftment in MF are incompletely characterized, we studied engraftment dynamics at our center.
Methods: A longitudinal cohort of 71 allogeneic HCT performed 2000-2019 with >50% after 2015 was evaluated.
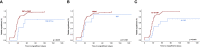
Results: Median time to neutrophil engraftment ≥0.5x109/l was +20 days post-transplant and associated with BM fibrosis, splenomegaly and infused CD34+ cell number. Engraftment dynamics were similar in primary vs. secondary MF and were independent of MF driver mutations in JAK2, CALR and MPL. Neutrophil engraftment occurred later upon haploidentical HCT with thiotepa-busulfan-fludarabine conditioning, post-transplant cyclophosphamide and G-CSF (TBF-PTCy/G-CSF) administered to 9.9% and 15.6% of patients in 2000-2019 and after 2015, respectively. Engraftment of platelets was similarly delayed, while reconstitution of reticulocytes was not affected.
Conclusions: Since MF is a rare hematologic malignancy, this data from a large number of HCT for MF is essential to substantiate that later neutrophil and platelet engraftment in MF relates both to host and treatment-related factors. Observations from this longitudinal cohort support that novel conditioning schemes administered also to rare entities such as MF, require detailed evaluation in larger, multi-center cohorts to assess also indicators of long-term graft function and overall outcome in patients with this infrequent hematopoietic neoplasm undergoing allogeneic transplantation.
Keywords: allogeneic hematopoietic cell transplantation; engraftment; myelofibrosis; myeloproliferative neoplasms; reconstitution.
Copyright © 2023 Jungius, Adam, Grosheintz, Medinger, Buser, Passweg, Halter and Meyer.
Conflict of interest statement
S.J. and F.C.A. hold shares in Novartis. S.C.M. has consulted for and received honoraria from Celgene/BMS, Novartis and GSK and receives research support from Ajax. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Fludarabine/Busulfan Conditioning-Based Allogeneic Hematopoietic Cell Transplantation for Myelofibrosis: Role of Ruxolitinib in Improving Survival Outcomes.Biol Blood Marrow Transplant. 2020 May;26(5):893-901. doi: 10.1016/j.bbmt.2020.01.010. Epub 2020 Jan 23. Biol Blood Marrow Transplant. 2020. PMID: 31982543
-
Thiotepa-busulfan-fludarabine (TBF) conditioning regimen in patients undergoing allogeneic hematopoietic cell transplantation for myelofibrosis: an outcome analysis from the Chronic Malignancies Working Party of the EBMT.Bone Marrow Transplant. 2021 Jul;56(7):1593-1602. doi: 10.1038/s41409-021-01222-z. Epub 2021 Feb 1. Bone Marrow Transplant. 2021. PMID: 33526919
-
Successful Outcome in Patients with Myelofibrosis Undergoing Allogeneic Donor Hematopoietic Cell Transplantation Using Reduced Doses of Post-Transplantation Cyclophosphamide: Challenges and Review of the Literature.Transplant Cell Ther. 2023 Jul;29(7):473.e1-473.e6. doi: 10.1016/j.jtct.2023.04.008. Epub 2023 Apr 20. Transplant Cell Ther. 2023. PMID: 37086849 Review.
-
Cryopreservation of Allogeneic Hematopoietic Cell Products During COVID-19 Pandemic: Graft Characterization and Engraftment Outcomes.Transplant Proc. 2023 Oct;55(8):1799-1809. doi: 10.1016/j.transproceed.2023.03.070. Epub 2023 Apr 21. Transplant Proc. 2023. PMID: 37210273 Free PMC article.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
References
-
- Lwin Y, Kennedy G, Gottlieb D, Kwan J, Ritchie D, Szer J, et al. . Australasian trends in allogeneic stem cell transplantation for myelofibrosis in the molecular era: A retrospective analysis from the australasian bone marrow transplant recipient registry. Biol Blood Marrow Transplant (2020) 26:2252–2261. doi: 10.1016/j.bbmt.2020.08.024 - DOI - PubMed
-
- McLornan D, Szydlo R, Koster L, Chalandon Y, Robin M, Wolschke C, et al. . Myeloablative and reduced-intensity conditioned allogeneic hematopoietic stem cell transplantation in myelofibrosis: A retrospective study by the chronic Malignancies working party of the european society for blood and marrow transplantation. Biol Blood Marrow Transplant (2019) 25:2167–2171. doi: 10.1016/j.bbmt.2019.06.034 - DOI - PubMed
-
- Ruiz-Argüelles GJ, Gómez-Almaguer D, David-Gomez-Rangel J, Vela-Ojeda J, Cantú-Rodríguez OG, Jaime-Pérez JC, et al. . Allogeneic hematopoietic stem cell transplantation with non-myeloablative conditioning in patients with acute myelogenous leukemia eligible for conventional allografting: a prospective study. Leuk Lymphoma (2004) 45:1191–5. doi: 10.1080/10428190310001642846 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

