Arbuscular mycorrhizal fungi impact the production of alkannin/shikonin and their derivatives in Alkanna tinctoria Tausch. grown in semi-hydroponic and pot cultivation systems
- PMID: 37637105
- PMCID: PMC10447974
- DOI: 10.3389/fmicb.2023.1216029
Arbuscular mycorrhizal fungi impact the production of alkannin/shikonin and their derivatives in Alkanna tinctoria Tausch. grown in semi-hydroponic and pot cultivation systems
Abstract
Introduction: Alkanna tinctoria Tausch. is a medicinal plant well-known to produce important therapeutic compounds, such as alkannin/shikonin and their derivatives (A/Sd). It associates with arbuscular mycorrhizal fungi (AMF), which are known, amongst others beneficial effects, to modulate the plant secondary metabolites (SMs) biosynthesis. However, to the best of our knowledge, no study on the effects of AMF strains on the growth and production of A/Sd in A. tinctoria has been reported in the literature.
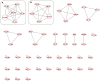
Methods: Here, three experiments were conducted. In Experiment 1, plants were associated with the GINCO strain Rhizophagus irregularis MUCL 41833 and, in Experiment 2, with two strains of GINCO (R. irregularis MUCL 41833 and Rhizophagus aggregatus MUCL 49408) and two native strains isolated from wild growing A. tinctoria (R. irregularis and Septoglomus viscosum) and were grown in a semi-hydroponic (S-H) cultivation system. Plants were harvested after 9 and 37 days in Experiment 1 and 9 days in Experiment 2. In Experiment 3, plants were associated with the two native AMF strains and with R. irregularis MUCL 41833 and were grown for 85 days in pots under greenhouse conditions. Quantification and identification of A/Sd were performed by HPLC-PDA and by HPLC-HRMS/MS, respectively. LePGT1, LePGT2, and GHQH genes involved in the A/Sd biosynthesis were analyzed through RT-qPCR.
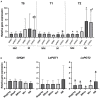
Results: In Experiment 1, no significant differences were noticed in the production of A/Sd. Conversely, in Experiments 2 and 3, plants associated with the native AMF R. irregularis had the highest content of total A/Sd expressed as shikonin equivalent. In Experiment 1, a significantly higher relative expression of both LePGT1 and LePGT2 was observed in plants inoculated with R. irregularis MUCL 41833 compared with control plants after 37 days in the S-H cultivation system. Similarly, a significantly higher relative expression of LePGT2 in plants inoculated with R. irregularis MUCL 41833 was noticed after 9 versus 37 days in the S-H cultivation system. In Experiment 2, a significant lower relative expression of LePGT2 was observed in native AMF R. irregularis inoculated plants compared to the control.
Discussion: Overall, our study showed that the native R. irregularis strain increased A/Sd production in A. tinctoria regardless of the growing system used, further suggesting that the inoculation of native/best performing AMF is a promising method to improve the production of important SMs.
Keywords: Alkanna tinctoria; alkannin/shikonin derivatives; arbuscular mycorrhizal fungi; native strains; semi-hydroponic cultivation system.
Copyright © 2023 Zhao, Cartabia, Garcés-Ruiz, Herent, Quetin-Leclercq, Ortiz, Declerck and Lalaymia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ahmad M., Leroy T., Krigas N., Temsch E. M., Weiss-Schneeweiss H., Lexer C., et al. (2021). Spatial and ecological drivers of genetic structure in Greek populations of Alkanna tinctoria (Boraginaceae), a polyploid medicinal herb. Front. Plant Sci. 12:706574. 10.3389/fpls.2021.706574 - DOI - PMC - PubMed
-
- Ahmad M., Varela A. A., Koletti A. E., Assimopoulou A. N., Declerck S., Schneider C., et al. (2022a). Transcriptional dynamics of Chitinophaga sp. strain R-73072-mediated alkannin/shikonin biosynthesis in Lithospermum officinale. Front. Microbiol. 13:978021. 10.3389/fmicb.2022.978021 - DOI - PMC - PubMed
-
- Andrade S. A. L., Malik S., Sawaya A. C. H. F., Bottcher A., Mazzafera P. (2013). Association with arbuscular mycorrhizal fungi influences alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum plants. Acta Physiol Plant. 35 867–880. 10.1007/s11738-012-1130-8 - DOI
LinkOut - more resources
Full Text Sources

