Development of a membrane-based Gi-CASE biosensor assay for profiling compounds at cannabinoid receptors
- PMID: 37637423
- PMCID: PMC10450933
- DOI: 10.3389/fphar.2023.1158091
Development of a membrane-based Gi-CASE biosensor assay for profiling compounds at cannabinoid receptors
Abstract
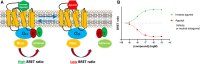
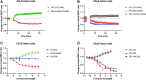
Introduction: The cannabinoid receptor (CBR) subtypes 1 (CB1R) and 2 (CB2R) are key components of the endocannabinoid system (ECS), playing a central role in the control of peripheral pain, inflammation and the immune response, with further roles in the endocrine regulation of food intake and energy balance. So far, few medicines targeting these receptors have reached the clinic, suggesting that a better understanding of the receptor signalling properties of existing tool compounds and clinical candidates may open the door to the development of more effective and safer treatments. Both CB1R and CB2R are Gαi protein-coupled receptors but detecting Gαi protein signalling activity reliably and reproducibly is challenging. This is due to the inherent variability in live cell-based assays and restrictions around the use of radioactive [35S]-GTPγS, a favoured technology for developing higher-throughput membrane-based Gαi protein activity assays. Methods: Here, we describe the development of a membrane-based Gαi signalling system, produced from membrane preparations of HEK293TR cells, stably overexpressing CB1R or CB2R, and components of the Gαi-CASE biosensor. This BRET-based system allows direct detection of Gαi signalling in both cells and membranes by monitoring bioluminescence resonance energy transfer (BRET) between the α and the βγ subunits. Cells and membranes were subject to increasing concentrations of reference cannabinoid compounds, with 10 μM furimazine added to generate RET signals, which were detected on a PHERAstar FSX plate reader, then processed using MARS software and analysed in GraphPad PRISM 9.2. Results: In membranes expressing the Gi-CASE biosensor, the cannabinoid ligands profiled were found to show agonist and inverse agonist activity. Agonist activity elicited a decrease in the BRET signal, indicative of receptor activation and G protein dissociation. Inverse agonist activity caused an increase in BRET signal, indicative of receptor inactivation, and the accumulation of inactive G protein. Our membrane-based Gi-CASE NanoBRET system successfully characterised the potency (pEC50) and efficacy (Emax) of CBR agonists and inverse agonists in a 384-well screening format. Values obtained were in-line with whole-cell Gi-CASE assays and consistent with literature values obtained in the GTPγS screening format. Discussion: This novel, membrane-based Gαi protein activation assay is applicable to other Gαi-coupled GPCRs, including orphan receptors, allowing real-time higher-throughput measurements of receptor activation.
Keywords: BRET—bioluminescence resonance energy transfer; G-protein signaling; GPCR signalling assay; Gαi signaling pathway; Gαi signalling assay; cannabinoid receptors (CBRs); inverse agonism.
Copyright © 2023 Scott-Dennis, Rafani, Yi, Perera, Harwood, Guba, Rufer, Grether, Veprintsev and Sykes.
Conflict of interest statement
Authors WG, AR, and UG was/were employed by F Hoffmann-La Roche Ltd. Authors DV and DS was/were employed by Z7 Biotech Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- AlKhelb D., Kirunda A., Ho T. C., Makriyannis A., Desai R. I. (2022). Effects of the cannabinoid CB1-receptor neutral antagonist AM4113 and antagonist/inverse agonist rimonabant on fentanyl discrimination in male rats. Drug Alcohol Depend. 240, 109646. 10.1016/j.drugalcdep.2022.109646 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

