Mechanochemical Phosphorylation of Acetylides Using Condensed Phosphates: A Sustainable Route to Alkynyl Phosphonates
- PMID: 37637745
- PMCID: PMC10451036
- DOI: 10.1021/acscentsci.3c00725
Mechanochemical Phosphorylation of Acetylides Using Condensed Phosphates: A Sustainable Route to Alkynyl Phosphonates
Abstract
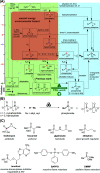
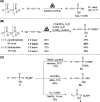
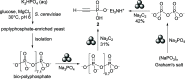
In pursuit of a more sustainable route to phosphorus-carbon (P-C) bond-containing chemicals, we herein report that phosphonates can be prepared by mechanochemical phosphorylation of acetylides using polyphosphates in a single step, redox-neutral process, bypassing white phosphorus (P4) and other high-energy, environmentally hazardous intermediates. Using sodium triphosphate (Na5P3O10) and acetylides, alkynyl phosphonates 1 can be isolated in yields of up to 32%, while reaction of sodium pyrophosphate (Na4P2O7) and sodium carbide (Na2C2) engendered, in an optimized yield of 63%, ethynyl phosphonate 2, an easily isolable compound that can be readily converted to useful organophosphorus chemicals. Highly condensed phosphates like Graham's salt and bioproduced polyphosphate were also found to be compatible after reducing the chain length by grinding with orthophosphate. These results demonstrate the possibility of accessing organophosphorus chemicals directly from condensed phosphates and may offer an opportunity to move toward a "greener" phosphorus industry.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Sustainable Production of Reduced Phosphorus Compounds: Mechanochemical Hydride Phosphorylation Using Condensed Phosphates as a Route to Phosphite.ACS Cent Sci. 2022 Mar 23;8(3):332-339. doi: 10.1021/acscentsci.1c01381. Epub 2022 Feb 14. ACS Cent Sci. 2022. PMID: 35350608 Free PMC article.
-
Comparison of various phosphate salts as the dietary phosphorus source on nephrocalcinosis and kidney function in rats.J Nutr Sci Vitaminol (Tokyo). 1999 Oct;45(5):595-608. doi: 10.3177/jnsv.45.595. J Nutr Sci Vitaminol (Tokyo). 1999. PMID: 10683811
-
Effects of partial and complete replacement of added phosphates with encapsulated phosphates on lipid oxidation inhibition in cooked ground meat during storage.Food Sci Technol Int. 2020 Apr;26(3):213-221. doi: 10.1177/1082013219881519. Epub 2019 Oct 11. Food Sci Technol Int. 2020. PMID: 31604384
-
The biological importance of organophosphorus compounds containing a carbon-phosphorus bond.Ciba Found Symp. 1977 Sep 13-15;(57):135-53. doi: 10.1002/9780470720387.ch8. Ciba Found Symp. 1977. PMID: 357117 Review.
-
Hydroxy- and Amino-Phosphonates and -Bisphosphonates: Synthetic Methods and Their Biological Applications.Front Chem. 2022 Jun 1;10:890696. doi: 10.3389/fchem.2022.890696. eCollection 2022. Front Chem. 2022. PMID: 35721002 Free PMC article. Review.
Cited by
-
A Greener Vision for P-C Bond Formation.ACS Cent Sci. 2023 Aug 10;9(8):1515-1517. doi: 10.1021/acscentsci.3c00943. eCollection 2023 Aug 23. ACS Cent Sci. 2023. PMID: 37637740 Free PMC article. No abstract available.
-
Direct conversion of various phosphate sources to a versatile P-X reagent [TBA][PO2X2] via redox-neutral halogenation.Nat Commun. 2025 Feb 26;16(1):2004. doi: 10.1038/s41467-025-57255-1. Nat Commun. 2025. PMID: 40011449 Free PMC article.
-
Closing the Loop: Low-Waste Phosphorus Functionalization Enabled by Simple Disulfides.ChemSusChem. 2025 Apr 1;18(7):e202401895. doi: 10.1002/cssc.202401895. Epub 2024 Nov 25. ChemSusChem. 2025. PMID: 39526941 Free PMC article.
References
-
- Svara J.; Weferling N.; Hofmann T.. Phosphorus Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd., 2006; Vol. 27; pp 19–49.
-
- Joly D.; Bouit P.-A.; Hissler M. Organophosphorus derivatives for electronic devices. J. Mater. Chem. C 2016, 4, 3686–3698. 10.1039/C6TC00590J. - DOI
-
- Demkowicz S.; Rachon J.; Daśko M.; Kozak W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101–7112. 10.1039/C5RA25446A. - DOI
LinkOut - more resources
Full Text Sources

