Paneth cell dysfunction in radiation injury and radio-mitigation by human α-defensin 5
- PMID: 37638013
- PMCID: PMC10448521
- DOI: 10.3389/fimmu.2023.1174140
Paneth cell dysfunction in radiation injury and radio-mitigation by human α-defensin 5
Abstract
Introduction: The mechanism underlying radiation-induced gut microbiota dysbiosis is undefined. This study examined the effect of radiation on the intestinal Paneth cell α-defensin expression and its impact on microbiota composition and mucosal tissue injury and evaluated the radio-mitigative effect of human α-defensin 5 (HD5).
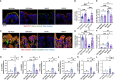
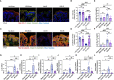
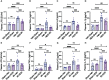
Methods: Adult mice were subjected to total body irradiation, and Paneth cell α-defensin expression was evaluated by measuring α-defensin mRNA by RT-PCR and α-defensin peptide levels by mass spectrometry. Vascular-to-luminal flux of FITC-inulin was measured to evaluate intestinal mucosal permeability and endotoxemia by measuring plasma lipopolysaccharide. HD5 was administered in a liquid diet 24 hours before or after irradiation. Gut microbiota was analyzed by 16S rRNA sequencing. Intestinal epithelial junctions were analyzed by immunofluorescence confocal microscopy and mucosal inflammatory response by cytokine expression. Systemic inflammation was evaluated by measuring plasma cytokine levels.
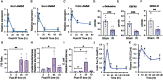
Results: Ionizing radiation reduced the Paneth cell α-defensin expression and depleted α-defensin peptides in the intestinal lumen. α-Defensin down-regulation was associated with the time-dependent alteration of gut microbiota composition, increased gut permeability, and endotoxemia. Administration of human α-defensin 5 (HD5) in the diet 24 hours before irradiation (prophylactic) significantly blocked radiation-induced gut microbiota dysbiosis, disruption of intestinal epithelial tight junction and adherens junction, mucosal barrier dysfunction, and mucosal inflammatory response. HD5, administered 24 hours after irradiation (treatment), reversed radiation-induced microbiota dysbiosis, tight junction and adherens junction disruption, and barrier dysfunction. Furthermore, HD5 treatment also prevents and reverses radiation-induced endotoxemia and systemic inflammation.
Conclusion: These data demonstrate that radiation induces Paneth cell dysfunction in the intestine, and HD5 feeding prevents and mitigates radiation-induced intestinal mucosal injury, endotoxemia, and systemic inflammation.
Keywords: GI-ARS; barrier function; defensins; intestine; irradiation; microbiome; tight junction.
Copyright © 2023 Shukla, Rao, Meena, Giorgianni, Lee, Raju, Shashikanth, Shekhar, Beranova, Balazs, Tigyi, Gosain and Rao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Human Defensin-5 Blocks Ethanol and Colitis-Induced Dysbiosis, Tight Junction Disruption and Inflammation in Mouse Intestine.Sci Rep. 2018 Nov 2;8(1):16241. doi: 10.1038/s41598-018-34263-4. Sci Rep. 2018. PMID: 30389960 Free PMC article.
-
Paneth Cell Dysfunction Mediates Alcohol-related Steatohepatitis Through Promoting Bacterial Translocation in Mice: Role of Zinc Deficiency.Hepatology. 2020 May;71(5):1575-1591. doi: 10.1002/hep.30945. Epub 2020 Mar 10. Hepatology. 2020. PMID: 31520476 Free PMC article.
-
TRPV6 deficiency attenuates stress and corticosterone-mediated exacerbation of alcohol-induced gut barrier dysfunction and systemic inflammation.Front Immunol. 2023 Jan 31;14:1093584. doi: 10.3389/fimmu.2023.1093584. eCollection 2023. Front Immunol. 2023. PMID: 36817471 Free PMC article.
-
Innate immune functions of α-defensins in the small intestine.Dig Dis. 2013;31(3-4):299-304. doi: 10.1159/000354681. Epub 2013 Nov 14. Dig Dis. 2013. PMID: 24246978 Review.
-
Enteric α-defensins on the verge of intestinal immune tolerance and inflammation.Semin Cell Dev Biol. 2019 Apr;88:138-146. doi: 10.1016/j.semcdb.2018.01.007. Epub 2018 Feb 1. Semin Cell Dev Biol. 2019. PMID: 29355606 Review.
Cited by
-
Human α-Defensin 51-9 and Human β-Defensin 2 Improve Metabolic Parameters and Gut Barrier Function in Mice Fed a Western-Style Diet.Int J Mol Sci. 2023 Sep 9;24(18):13878. doi: 10.3390/ijms241813878. Int J Mol Sci. 2023. PMID: 37762180 Free PMC article.
-
Defensins: Exploring Their Opposing Roles in Colorectal Cancer Progression.Cancers (Basel). 2024 Jul 23;16(15):2622. doi: 10.3390/cancers16152622. Cancers (Basel). 2024. PMID: 39123348 Free PMC article. Review.
-
Application of a derivative of human defensin 5 to treat ionizing radiation-induced enterogenic infection.J Radiat Res. 2024 Mar 22;65(2):194-204. doi: 10.1093/jrr/rrad104. J Radiat Res. 2024. PMID: 38264835 Free PMC article.
-
Paneth Cells: Dispensable yet Irreplaceable for the Intestinal Stem Cell Niche.Cell Mol Gastroenterol Hepatol. 2025;19(4):101443. doi: 10.1016/j.jcmgh.2024.101443. Epub 2024 Dec 19. Cell Mol Gastroenterol Hepatol. 2025. PMID: 39708920 Free PMC article. Review.
-
Spatial Proteomics Reveals Alcohol-Induced Damages to the Crypts and Villi of the Mouse Small Intestine.J Proteome Res. 2024 May 3;23(5):1801-1809. doi: 10.1021/acs.jproteome.4c00037. Epub 2024 Apr 24. J Proteome Res. 2024. PMID: 38655769 Free PMC article.
References
-
- Carbonero F, Mayta-Apaza AC, Yu JZ, Lindeblad M, Lyubimov A, Neri F, et al. A comparative analysis of gut microbiota disturbances in the gottingen minipig and rhesus macaque models of acute radiation syndrome following bioequivalent radiation exposures. Radiat Environ Biophys (2018) 57(4):419–26. doi: 10.1007/s00411-018-0759-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

