Brensocatib (an oral, reversible inhibitor of dipeptidyl peptidase-1) attenuates disease progression in two animal models of rheumatoid arthritis
- PMID: 37638021
- PMCID: PMC10451067
- DOI: 10.3389/fimmu.2023.1231047
Brensocatib (an oral, reversible inhibitor of dipeptidyl peptidase-1) attenuates disease progression in two animal models of rheumatoid arthritis
Abstract
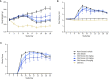
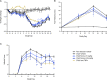
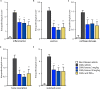
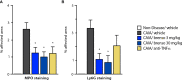
Rheumatoid arthritis (RA) is a painful and incurable disease characterized by chronic joint inflammation and a progressive destruction of cartilage and bone. Although current treatments have improved clinical outcomes for some patients, the high relapse rates and sizeable proportion of non-responders emphasize the need for further research. Arthritic joints are massively infiltrated by neutrophils, which influence inflammatory and immune processes by releasing cytokines, chemokines, eicosanoids, and neutrophil serine proteases (NSPs) - all of which are known to contribute to RA initiation and progression. Active NSPs are generated from zymogens at the promyelocytic stage of neutrophil differentiation under the action of dipeptidyl peptidase 1 (DPP-1) and DPP-1 knockout mice are resistant to the development of arthritis. Thus, DPP-1 inhibition represents a promising therapeutic approach in RA. In this study, we assessed the efficacy of a potent and highly selective DPP-1 inhibitor, brensocatib, in two well established RA models - rat collagen-induced arthritis (CIA) and mouse collagen antibody-induced arthritis (CAIA). In both models, brensocatib at 3 and 30 mg/kg/day significantly reduced bone marrow NSP levels, in keeping with prior pharmacodynamic studies in rodents. More importantly, brensocatib treatment significantly improved disease score at both dosages in both rodent models. In the mouse CAIA model, brensocatib even proved at least as potent as anti-TNF antibodies in diminishing both the histopathological score and neutrophil infiltration into arthritic joints. Together, these results show that brensocatib alters RA disease progression in rodents and supports the need for its further evaluation as a potential therapeutic option, or to complement existing RA treatments.
Keywords: DPP-1; cathepsin C; inflammation; migration; neutrophil serine proteases; neutrophils.
Copyright © 2023 McDonald, Leifer, Basso, Lasala, Li, Chen, Zhang, Perkins and Cipolla.
Conflict of interest statement
All authors were employed by Insmed Incorporated at the time of the study conduct and/or preparation of the manuscript.
Figures

References
-
- Brennan FM, Zachariae CO, Chantry D, Larsen CG, Turner M, Maini RN, et al. . Detection of interleukin-8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol (1990) 20:2141–4. doi: 10.1002/eji.1830200938 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

