Lipocalin 2 receptors: facts, fictions, and myths
- PMID: 37638032
- PMCID: PMC10451079
- DOI: 10.3389/fimmu.2023.1229885
Lipocalin 2 receptors: facts, fictions, and myths
Abstract
The human 25-kDa Lipocalin 2 (LCN2) was first identified and purified as a protein that in part is associated with gelatinase from neutrophils. This protein shows a high degree of sequence similarity with the deduced sequences of rat α2-microglobulin-related protein and the mouse protein 24p3. Based on its typical lipocalin fold, which consists of an eight-stranded, anti-parallel, symmetrical β-barrel fold structure it was initially thought that LCN2 is a circulating protein functioning as a transporter of small lipophilic molecules. However, studies in Lcn2 null mice have shown that LCN2 has bacteriostatic properties and plays a key role in innate immunity by sequestering bacterial iron siderophores. Numerous reports have further shown that LCN2 is involved in the control of cell differentiation, energy expenditure, cell death, chemotaxis, cell migration, and many other biological processes. In addition, important roles for LCN2 in health and disease have been identified in Lcn2 null mice and multiple molecular pathways required for regulation of Lcn2 expression have been identified. Nevertheless, although six putative receptors for LCN2 have been proposed, there is a fundamental lack in understanding of how these cell-surface receptors transmit and amplify LCN2 to the cell. In the present review we summarize the current knowledge on LCN2 receptors and discuss inconsistencies, misinterpretations and false assumptions in the understanding of these potential LCN2 receptors.
Keywords: LRP2; MC4R; Megalin; NGALR; SLC22A17; inflammation; iron.
Copyright © 2023 Schröder, Gasterich, Weiskirchen and Weiskirchen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

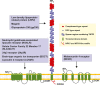
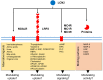



References
-
- Hraba-Renevey S, Türler H, Kress M, Salomon C, Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene (1989) 4(5):601–8. - PubMed
-
- RCSB . Protein Data Bank (PDB). Available at: https://www.rcsb.org/ (Accessed 11 May 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

