Fluorescent nano- and microparticles for sensing cellular microenvironment: past, present and future applications
- PMID: 37638162
- PMCID: PMC10448310
- DOI: 10.1039/d3na00218g
Fluorescent nano- and microparticles for sensing cellular microenvironment: past, present and future applications
Abstract
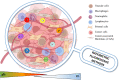
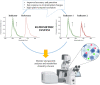
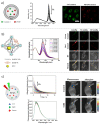
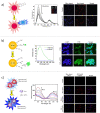
The tumor microenvironment (TME) demonstrates distinct hallmarks, including acidosis, hypoxia, reactive oxygen species (ROS) generation, and altered ion fluxes, which are crucial targets for early cancer biomarker detection, tumor diagnosis, and therapeutic strategies. Various imaging and sensing techniques have been developed and employed in both research and clinical settings to visualize and monitor cellular and TME dynamics. Among these, ratiometric fluorescence-based sensors have emerged as powerful analytical tools, providing precise and sensitive insights into TME and enabling real-time detection and tracking of dynamic changes. In this comprehensive review, we discuss the latest advancements in ratiometric fluorescent probes designed for the optical mapping of pH, oxygen, ROS, ions, and biomarkers within the TME. We elucidate their structural designs and sensing mechanisms as well as their applications in in vitro and in vivo detection. Furthermore, we explore integrated sensing platforms that reveal the spatiotemporal behavior of complex tumor cultures, highlighting the potential of high-resolution imaging techniques combined with computational methods. This review aims to provide a solid foundation for understanding the current state of the art and the future potential of fluorescent nano- and microparticles in the field of cellular microenvironment sensing.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- American Cancer Society, Cancer Facts & Figures, 2022
-
- Siegel R. L. Miller K. D. Wagle N. S. Jemal A. Cancer C. A. J. Clin. 2023;73:17–48. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

