Improving Surgical Informed Consent: Unanswered Questions
- PMID: 37638239
- PMCID: PMC10455139
- DOI: 10.1097/AS9.0000000000000030
Improving Surgical Informed Consent: Unanswered Questions
Abstract
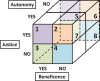
Objective: This study reviews randomized clinical trials that have attempted to improve the process of informed consent. Consent should be guided by the ethical imperatives of autonomy, beneficence, and social justice.
Summary background: Informed consent is constantly evolving. Yet our review of the randomized trials done to improve the surgical informed consent process raises a number of questions: How does one define surgical informed consent? What interventions have been tried to measure and improve informed consent? Have the interventions in informed consent actually led to improvements? What efforts have been made to improve informed consent? And what steps can be taken to improve the process further?
Methods: A literature search for randomized controlled trials (RCTs)on informed consent identified 70 trials. Demographics, interventions, assessments, and a semi-quantitative summary of the findings were tabulated. The assessments done in the RCTs, show the surrogate for patient autonomy was comprehension; for beneficence, satisfaction and mental state (anxiety or depression); and, for social justice, language, literacy, learning needs, and cost.
Results: There were 4 basic categories of interventions: printed matter; non-interactive audiovisual tools; interactive multimedia; and a smaller group defying easy description. Improvement was documented in 46 of the 65 trials that studied comprehension. Thirteen of 33 trials showed improved satisfaction. Three of 30 studies showed an increase in anxiety. Few studies tried to assess primary language or literacy, and none looked at learning needs or cost.
Conclusions: No single study improved all 3 principles of informed consent. Validated interventions and assessments were associated with greater impact on outcomes. All 3 ethical principles should be assessed; autonomy (as comprehension), beneficence (as satisfaction, anxiety), and social justice. Not enough consideration has been given to social justice; appropriate language translation, standardized reading levels, assessment of learning needs, and cost to the individual are all important elements worthy of future study.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Disclosure: The authors declare that they have nothing to disclose.
Figures
References
-
- President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Vol. 1: report: making health care decisions: the ethical and legal implications of informed consent in the patient-practitioner relationship;1983 ASI 16088-1.1. 1983.
-
- Project of the ABIM Foundation, ACP–ASIM Foundation, and European Federation of Internal Medicine. Medical professionalism in the New Millennium: a physician charter. Ann Intern Med. 2002; 136:243. - PubMed
-
- Shamseer L, Moher D, Clarke M, et al.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015; 350:g7647. - PubMed
-
- Kostick KM, Bruce CR, Minard CG, et al. A multisite randomized controlled trial of a patient-centered ventricular assist device decision aid (VADDA trial). J Card Fail. 2018; 24:661–671. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

