"Of mice and men": the relevance of Cometin and Erythropoietin origin for its effects on murine spiral ganglion neuron survival and neurite outgrowth in vitro
- PMID: 37638326
- PMCID: PMC10450246
- DOI: 10.3389/fnins.2023.1224463
"Of mice and men": the relevance of Cometin and Erythropoietin origin for its effects on murine spiral ganglion neuron survival and neurite outgrowth in vitro
Abstract
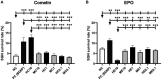
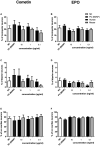
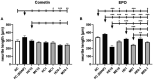
Neurotrophic factors (NTF) play key roles in the survival of neurons, making them promising candidates for therapy of neurodegenerative diseases. In the case of the inner ear, sensorineural hearing loss (SNHL) is characterized over time by a degeneration of the primary auditory neurons, the spiral ganglion neurons (SGN). It is well known that selected NTF can protect SGN from degeneration, which positively influences the outcome of cochlear implants, the treatment of choice for patients with profound to severe SNHL. However, the outcome of studies investigating protective effects of NTF on auditory neurons are in some cases of high variability. We hypothesize that the factor origin may be one aspect that affects the neuroprotective potential. The aim of this study was to investigate the neuroprotective potential of human and mouse Erythropoietin (EPO) and Cometin on rat SGN. SGN were isolated from neonatal rats (P 2-5) and cultured in serum-free medium. EPO and Cometin of mouse and human origin were added in concentrations of 0.1, 1, and 10 ng/mL and 0.1, 1, and 10 μg/mL, respectively. The SGN survival rate and morphology, and the neurite outgrowth were determined and compared to negative (no additives) and positive (brain-derived neurotrophic factor, BDNF) controls. A neuroprotective effect of 10 μg/mL human Cometin comparable to that obtained with BDNF was observed in the SGN-culture. In contrast, mouse Cometin was ineffective. A similar influence of 10 μg/mL human and mouse and 1 μg/mL human Cometin on the length of regenerated neurites compared to BDNF was also detected. No other Cometin-conditions, and none of the EPO-conditions tested had neuroprotective or neuritogenic effects or influenced the neuronal morphology of the SGN. The neuroprotective effect of 10 μg/mL human Cometin on SGN indicates it is a potentially interesting protein for the supportive treatment of inner ear disorders. The finding that mouse Cometin had no effect on the SGN in the parallel-performed experiments underlines the importance of species origin of molecules being screened for therapeutic purpose.
Keywords: cochlea; drug delivery; inner ear; neurite outgrowth; neuroprotection; pharmacotherapy; species specific.
Copyright © 2023 Schwieger, Gao, Lenarz, Munro, Petersen and Scheper.
Conflict of interest statement
Authors GM and KP were employed by company Hoba Therapeutics ApS. All authors declare that this study received funding from Hoba Therapeutics ApS. This funder had the following involvement in the study: contribution of reagents and materials, study design, and preparation of the manuscript.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

