Spectral flow cytometry identifies distinct nonneoplastic plasma extracellular vesicle phenotype in glioblastoma patients
- PMID: 37638345
- PMCID: PMC10457026
- DOI: 10.1093/noajnl/vdad082
Spectral flow cytometry identifies distinct nonneoplastic plasma extracellular vesicle phenotype in glioblastoma patients
Abstract
Background: Glioblastoma (GBM) is the most common malignant brain tumor and has a poor prognosis. Imaging findings at diagnosis and in response to treatment are nonspecific. Developing noninvasive assays to augment imaging would be helpful. Plasma extracellular vesicles (EVs) are a promising biomarker source for this. Here, we develop spectral flow cytometry techniques that demonstrate differences in bulk plasma EV phenotype between GBM patients and normal donors that could serve as the basis of a liquid biopsy.
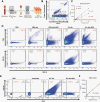
Methods: Plasma EVs were stained for EV-associated tetraspanins (CD9/CD63/CD81), markers indicating cell of origin (CD11b/CD31/CD41a/CD45), and actin/phalloidin (to exclude cell debris). EVs were analyzed using spectral flow cytometry. Multiparametric analysis using t-distributed stochastic neighbor embedding (t-SNE) and self-organizing maps on flow cytometry data (FlowSOM) was performed comparing GBM and normal donor (ND) plasma EVs.
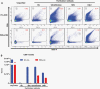
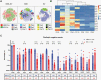
Results: Size exclusion chromatography plus spectral-based flow cytometer threshold settings enriched plasma EVs while minimizing background noise. GBM patients had increased CD9+, CD63+, CD81+, and myeloid-derived (CD11b+) EVs. Multiparametric analysis demonstrated distinct surface marker expression profiles in GBM plasma EVs compared to ND EVs. Fifteen plasma EV sub-populations differing in size and surface marker expression were identified, six enriched in GBM patients and two in normal donors.
Conclusions: Multiparametric analysis demonstrates that GBM patients have a distinct nonneoplastic plasma EV phenotype compared to ND. This simple rapid analysis can be performed without purifying tumor EVs and may serve as the basis of a liquid biopsy.
Keywords: extracellular vesicles; flow cytometry; glioblastoma; liquid biopsy.
© The Author(s) 2023. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
no authors have relevant conflicts of interest to disclose.
Figures


References
-
- Ostrom QT, Gittleman H, Stetson L, Virk S, Barnholtz-Sloan JS. Epidemiology of intracranial gliomas. Prog Neurol Surg. 2018;30:1–11. - PubMed
-
- Nam JY, de Groot JF.. Treatment of glioblastoma. J Oncol Pract. 2017;13(10):629–638. - PubMed
-
- Marenco-Hillembrand L, Wijesekera O, Suarez-Meade P, et al. . Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J Neurooncol. 2020;147(2):297–307. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
