Over diagnosis of bradykinin angioedema in patients treated with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers
- PMID: 37638360
- PMCID: PMC10458346
- DOI: 10.1016/j.waojou.2023.100809
Over diagnosis of bradykinin angioedema in patients treated with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers
Abstract
Background: Bradykinin angioedemas are a potentially serious side effect of angiotensin-converting enzyme inhibitors (ACEI) and more controversially of angiotensin II receptor blockers (ARB). Their challenging diagnosis is based on the absence of any recurrence after more than 6 months of drug discontinuation; otherwise mast-cell driven angioedemas as a differential diagnosis must be considered.
Objective: The aim of this study was to determine the prevalence of recurrent angioedema in patients referred for ACEI/ARB-induced bradykinin angioedema, after more than 6 months of drug discontinuation.
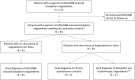
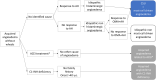
Methods: We included ACEI/ARB-treated patients referred for angioedema(s) without hives and unresponsive to antihistamines, after they discontinued ACEI/ARB for at least 6 months. Any C1-inhibitor deficiency was excluded. The primary endpoint was the prevalence of patients with recurrent angioedema after more than 6 months of drug discontinuation and/or developing hives during follow-up. The secondary endpoint was the identification of epidemiological factors associated with any final diagnosis.
Results: Thirty-eight of 93 patients (41%) with a suspicion of ACEI/ARB-induced bradykinin angioedema still had recurrent angioedema (n = 27) or developed hives (n = 2) or both (n = 9) after 6 months of drug discontinuation. Good response to icatibant and facial but not oral localization were predictive for the final diagnosis of ACEI/ARB-induced bradykinin angioedema and mast-cell driven angioedema, respectively.
Conclusion: In patients referred for acquired angioedema without wheals occurring during ACEI/ARB therapy, 59% finally had a diagnosis of ACEI/ARB-induced bradykinin angioedema whereas 41% were rather diagnosed with mast-cell driven angioedema. The overdiagnosis of ACEI/ARB-induced bradykinin angioedema may deteriorate the management of severe cardiovascular conditions.
Keywords: Angioedema; Angiotensin converting enzyme inhibitors; Bradykinin; Mast-cell; Urticaria.
© 2023 Published by Elsevier Inc. on behalf of World Allergy Organization.
Conflict of interest statement
None.
Figures

References
-
- Hoover T., Lippmann M., Grouzmann E., Marceau F., Herscu P. Angiotensin converting enzyme inhibitor induced angio-oedema: a review of the pathophysiology and risk factors. Clin Exp Allergy. 2010;40(1):50–61. - PubMed
-
- Dicpinigaitis P.V. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006 Jan 1;129(1, Supplement):169S–173S. - PubMed
-
- Caldeira D., David C., Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors. Am J Cardiovasc Drugs. 2012 Aug 1;12(4):263–277. - PubMed
LinkOut - more resources
Full Text Sources

