Microglia-Astrocyte Communication in Alzheimer's Disease
- PMID: 37638434
- PMCID: PMC10578295
- DOI: 10.3233/JAD-230199
Microglia-Astrocyte Communication in Alzheimer's Disease
Abstract
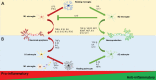
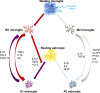
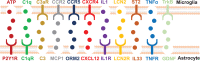
Microglia and astrocytes are regarded as active participants in the central nervous system under various neuropathological conditions, including Alzheimer's disease (AD). Both microglia and astrocyte activation have been reported to occur with a spatially and temporarily distinct pattern. Acting as a double-edged sword, glia-mediated neuroinflammation may be both detrimental and beneficial to the brain. In a variety of neuropathologies, microglia are activated before astrocytes, which facilitates astrocyte activation. Yet reactive astrocytes can also prevent the activation of adjacent microglia in addition to helping them become activated. Studies describe changes in the genetic profile as well as cellular and molecular responses of these two types of glial cells that contribute to dysfunctional immune crosstalk in AD. In this paper, we construct current knowledge of microglia-astrocyte communication, highlighting the multifaceted functions of microglia and astrocytes and their role in AD. A thorough comprehension of microglia-astrocyte communication could hasten the creation of novel AD treatment approaches.
Keywords: Alzheimer’s disease; astrocyte; cellular crosstalk; microglia; neuroinflammation.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures
Similar articles
-
Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer's disease.Inflammopharmacology. 2019 Aug;27(4):663-677. doi: 10.1007/s10787-019-00580-x. Epub 2019 Mar 14. Inflammopharmacology. 2019. PMID: 30874945 Review.
-
Tryptophan Metabolism in Alzheimer's Disease with the Involvement of Microglia and Astrocyte Crosstalk and Gut-Brain Axis.Aging Dis. 2024 Oct 1;15(5):2168-2190. doi: 10.14336/AD.2024.0134. Aging Dis. 2024. PMID: 38916729 Free PMC article. Review.
-
Deleterious Alteration of Glia in the Brain of Alzheimer's Disease.Int J Mol Sci. 2020 Sep 12;21(18):6676. doi: 10.3390/ijms21186676. Int J Mol Sci. 2020. PMID: 32932623 Free PMC article. Review.
-
Isolation of glia from Alzheimer's mice reveals inflammation and dysfunction.Neurobiol Aging. 2014 Dec;35(12):2746-2760. doi: 10.1016/j.neurobiolaging.2014.06.004. Epub 2014 Jun 14. Neurobiol Aging. 2014. PMID: 25002035
-
Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates.J Neuroinflammation. 2021 Jun 3;18(1):124. doi: 10.1186/s12974-021-02158-3. J Neuroinflammation. 2021. PMID: 34082772 Free PMC article.
Cited by
-
From Fundamentals to Innovation in Alzheimer's Disease: Molecular Findings and Revolutionary Therapies.Int J Mol Sci. 2024 Nov 16;25(22):12311. doi: 10.3390/ijms252212311. Int J Mol Sci. 2024. PMID: 39596378 Free PMC article. Review.
-
Astrocyte-microglia crosstalk in subarachnoid hemorrhage: mechanisms and treatments.Front Immunol. 2025 Jun 30;16:1547858. doi: 10.3389/fimmu.2025.1547858. eCollection 2025. Front Immunol. 2025. PMID: 40661949 Free PMC article. Review.
-
Inhibition of astrocyte signaling leads to sex-specific changes in microglia phenotypes in a diet-based model of cerebral small vessel disease.J Neuroinflammation. 2025 Aug 9;22(1):202. doi: 10.1186/s12974-025-03523-2. J Neuroinflammation. 2025. PMID: 40783543 Free PMC article.
-
The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology.Antioxidants (Basel). 2024 Oct 8;13(10):1208. doi: 10.3390/antiox13101208. Antioxidants (Basel). 2024. PMID: 39456461 Free PMC article. Review.
-
Clusterin Regulates the Mechanisms of Neuroinflammation and Neuronal Circuit Impairment in Alzheimer's Disease.Int J Mol Sci. 2025 Jul 28;26(15):7271. doi: 10.3390/ijms26157271. Int J Mol Sci. 2025. PMID: 40806404 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

