Invasive Squamous Cell Carcinoma of the Cervix: A Review of Morphological Appearances Encountered in Human Papillomavirus-associated and Papillomavirus-independent Tumors and Precursor Lesions
- PMID: 37638549
- PMCID: PMC10841279
- DOI: 10.1097/PAP.0000000000000411
Invasive Squamous Cell Carcinoma of the Cervix: A Review of Morphological Appearances Encountered in Human Papillomavirus-associated and Papillomavirus-independent Tumors and Precursor Lesions
Abstract
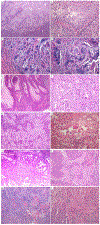
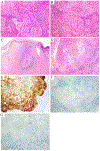
Cervical cancer is the fourth most common cancer among women globally. Historically, human papillomavirus (HPV) infection was considered necessary for the development of both precursor and invasive epithelial tumors of the cervix; however, studies in the last decade have shown that a significant proportion of cervical carcinomas are HPV-independent (HPVI). The 2020 World Health Organization (WHO) Classification of Female Genital Tumors separates both squamous cell carcinomas (SCCs) and endocervical adenocarcinomas (ECAs) by HPV status into HPV-associated (HPVA) and HPVI tumors. The classification further indicates that, in contrast to endocervical adenocarcinomas, HPVI and HPVA SCCs cannot be distinguished by morphological criteria alone and suggests that HPV testing or correlates thereof are required for correct classification. Moreover, while HPVA SCC precursor lesions (ie, high-grade squamous intraepithelial lesion) are well known and characterized, precursors to HPVI SCCs have only been described recently in a small number of cases. We studied 670 cases of SCCs from the International Squamous Cell Carcinoma Project (ISCCP) to analyze the reproducibility of recognition of invasive SCC growth patterns, presence of lymphovascular space invasion, tumor grade, and associations with patient outcomes. Consistent with previous studies, we found histologic growth patterns and tumor types had limited prognostic implications. In addition, we describe the wide morphologic spectrum of HPVA and HPVI SCCs and their precursor lesions, including tumor growth patterns, particular and peculiar morphologic features that can lead to differential diagnoses, and the role of ancillary studies in the diagnosis of these tumors.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Outside the current work, A.I. reports consulting fees from Mylan. The remaining authors have no conflicts of interest to disclose.
Figures
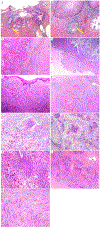
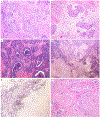

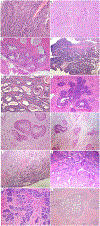
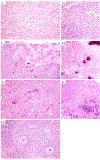

References
-
- WHO Classification of Tumours Female Genital Tumours. 5 ed. WHO Classification of Tumours. International Agency for Research on Cancer; 2020.
-
- Kurman RJ, Carcangiu ML, Herrington CS. World Health Organisation classification of tumours of the female reproductive organs. 4 ed. World Health Organization classification of tumours Lyon: International agency for research on cancer; 2014.

