RAP80 phase separation at DNA double-strand break promotes BRCA1 recruitment
- PMID: 37638744
- PMCID: PMC10570032
- DOI: 10.1093/nar/gkad686
RAP80 phase separation at DNA double-strand break promotes BRCA1 recruitment
Abstract
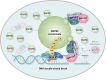
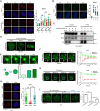
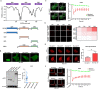
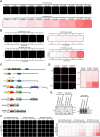
RAP80 has been characterized as a component of the BRCA1-A complex and is responsible for the recruitment of BRCA1 to DNA double-strand breaks (DSBs). However, we and others found that the recruitment of RAP80 and BRCA1 were not absolutely temporally synchronized, indicating that other mechanisms, apart from physical interaction, might be implicated. Recently, liquid-liquid phase separation (LLPS) has been characterized as a novel mechanism for the organization of key signaling molecules to drive their particular cellular functions. Here, we characterized that RAP80 LLPS at DSB was required for RAP80-mediated BRCA1 recruitment. Both cellular and in vitro experiments showed that RAP80 phase separated at DSB, which was ascribed to a highly disordered region (IDR) at its N-terminal. Meanwhile, the Lys63-linked poly-ubiquitin chains that quickly formed after DSBs occur, strongly enhanced RAP80 phase separation and were responsible for the induction of RAP80 condensation at the DSB site. Most importantly, abolishing the condensation of RAP80 significantly suppressed the formation of BRCA1 foci, encovering a pivotal role of RAP80 condensates in BRCA1 recruitment and radiosensitivity. Together, our study disclosed a new mechanism underlying RAP80-mediated BRCA1 recruitment, which provided new insight into the role of phase separation in DSB repair.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Darling A.L., Liu Y., Oldfield C.J., Uversky V.N.. Intrinsically disordered proteome of Human membrane-less organelles. Proteomics. 2018; 18:e1700193. - PubMed
-
- Uversky V.N., Gillespie J.R., Fink A.L. Why are “natively unfolded” proteins unstructured under physiologic conditions?. Proteins. 2000; 41:415–427. - PubMed
Grants and funding
- 2022YFA1105300/National Key Research and Development Program of China
- 82225040/National Science Fund for Distinguished Young Scholars
- 82122057/National Science Fund for Excellent Young Scholars
- 82103770/Natural Science Foundation of China
- 2021B1515020022/Guangdong Natural Science Funds for Distinguished Young Scholars
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

