Elevated nocturnal respiratory rates in the mitochondria of CAM plants: current knowledge and unanswered questions
- PMID: 37638861
- PMCID: PMC10799998
- DOI: 10.1093/aob/mcad119
Elevated nocturnal respiratory rates in the mitochondria of CAM plants: current knowledge and unanswered questions
Abstract
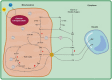
Crassulacean acid metabolism (CAM) is a metabolic adaptation that has evolved convergently in 38 plant families to aid survival in water-limited niches. Whilst primarily considered a photosynthetic adaptation, CAM also has substantial consequences for nocturnal respiratory metabolism. Here, we outline the history, current state and future of nocturnal respiration research in CAM plants, with a particular focus on the energetics of nocturnal respiratory oxygen consumption. Throughout the 20th century, research interest in nocturnal respiration occurred alongside initial discoveries of CAM, although the energetic and mechanistic implications of nocturnal oxygen consumption and links to the operation of the CAM cycle were not fully understood. Recent flux balance analysis (FBA) models have provided new insights into the role that mitochondria play in the CAM cycle. Several FBA models have predicted that CAM requires elevated nocturnal respiratory rates, compared to C3 species, to power vacuolar malic acid accumulation. We provide physiological data, from the genus Clusia, to corroborate these modelling predictions, thereby reinforcing the importance of elevated nocturnal respiratory rates for CAM. Finally, we outline five unanswered questions pertaining to nocturnal respiration which must be addressed if we are to fully understand and utilize CAM plants in a hotter, drier world.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures




Similar articles
-
Forty years of research into crassulacean acid metabolism in the genus Clusia: anatomy, ecophysiology and evolution.Ann Bot. 2023 Nov 25;132(4):739-752. doi: 10.1093/aob/mcad039. Ann Bot. 2023. PMID: 36891814 Free PMC article. Review.
-
CAM photosynthesis: the acid test.New Phytol. 2022 Jan;233(2):599-609. doi: 10.1111/nph.17790. Epub 2021 Nov 5. New Phytol. 2022. PMID: 34637529 Free PMC article.
-
Dissecting succulence: Crassulacean acid metabolism and hydraulic capacitance are independent adaptations in Clusia leaves.Plant Cell Environ. 2023 May;46(5):1472-1488. doi: 10.1111/pce.14539. Epub 2023 Jan 18. Plant Cell Environ. 2023. PMID: 36624682
-
Metabolic modelling identifies mitochondrial Pi uptake and pyruvate efflux as key aspects of daytime metabolism and proton homeostasis in crassulacean acid metabolism leaves.New Phytol. 2024 Oct;244(1):159-175. doi: 10.1111/nph.20032. Epub 2024 Aug 7. New Phytol. 2024. PMID: 39113419
-
Engineering Crassulacean Acid Metabolism in C3 and C4 Plants.Cold Spring Harb Perspect Biol. 2024 Apr 1;16(4):a041674. doi: 10.1101/cshperspect.a041674. Cold Spring Harb Perspect Biol. 2024. PMID: 38052496 Review.
Cited by
-
Crassulacean acid metabolism (CAM) at the crossroads: a special issue to honour 50 years of CAM research by Klaus Winter.Ann Bot. 2023 Nov 25;132(4):553-561. doi: 10.1093/aob/mcad160. Ann Bot. 2023. PMID: 37856823 Free PMC article. No abstract available.
-
Synthetic crassulacean acid metabolism (SynCAM) for improving water-use efficiency in plants.Philos Trans R Soc Lond B Biol Sci. 2025 May 29;380(1927):20240249. doi: 10.1098/rstb.2024.0249. Epub 2025 May 29. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40439297 Review.
References
-
- Aoki K, Nishida K.. 1984. ATPase activity associated with vacuoles and tonoplast vesicles isolated from the CAM plant, Kalanchoë daigremontiana. Physiologia Plantarum 60: 21–25. doi: 10.1111/j.1399-3054.1984.tb04243.x. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

