Systematic vitamin D supplementation is associated with improved outcomes and reduced thyroid adverse events in patients with cancer treated with immune checkpoint inhibitors: results from the prospective PROVIDENCE study
- PMID: 37638980
- PMCID: PMC10576732
- DOI: 10.1007/s00262-023-03522-3
Systematic vitamin D supplementation is associated with improved outcomes and reduced thyroid adverse events in patients with cancer treated with immune checkpoint inhibitors: results from the prospective PROVIDENCE study
Abstract
Background: Hypovitaminosis D can have a negative prognostic impact in patients with cancer. Vitamin D has a demonstrated role in T-cell-mediated immune activation. We hypothesized that systematic vitamin D repletion could impact clinical outcomes in patients with cancer receiving immune-checkpoint inhibitors (ICIs).
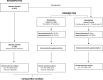
Methods: We planned a prospective observational study (PROVIDENCE) to assess serum vitamin D levels in patients with advanced cancer receiving ICIs (cohort 1 at treatment initiation, cohort 2 during treatment) and the impact of systematic repletion on survival and toxicity outcomes. In an exploratory analysis, we compared the clinical outcomes of cohort 1 with a control cohort of patients followed at the participating centers who did not receive systematic vitamin D repletion.
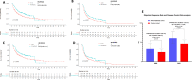
Results: Overall, 164 patients were prospectively recruited in the PROVIDENCE study. In cohort 1, consisting of 101 patients with 94.1% hypovitaminosis (≤ 30 ng/ml) at baseline, adequate repletion with cholecalciferol was obtained in 70.1% at the three months re-assessment. Cohort 2 consisted of 63 patients assessed for vitamin D at a median time of 3.7 months since immunotherapy initiation, with no patients having adequate levels (> 30 ng/ml). Even in cohort 2, systematic supplementation led to adequate levels in 77.8% of patients at the three months re-assessment. Compared to a retrospective control group of 238 patients without systematic vitamin D repletion, PROVIDENCE cohort 1 showed longer overall survival (OS, p = 0.013), time to treatment failure (TTF, p = 0.017), and higher disease control rate (DCR, p = 0.016). The Inverse Probability of Treatment Weighing (IPTW) fitted multivariable Cox regression confirmed the significantly decreased risk of death (HR 0.55, 95%CI: 0.34-0.90) and treatment discontinuation (HR 0.61, 95%CI: 0.40-0.91) for patients from PROVIDENCE cohort 1 in comparison to the control cohort. In the context of longer treatment exposure, the cumulative incidence of any grade immune-related adverse events (irAEs) was higher in the PROVIDENCE cohort 1 compared to the control cohort. Nevertheless, patients from cohort 1 experienced a significantly decreased risk of all grade thyroid irAEs than the control cohort (OR 0.16, 95%CI: 0.03-0.85).
Conclusion: The PROVIDENCE study suggests the potential positive impact of early systematic vitamin D supplementation on outcomes of patients with advanced cancer receiving ICIs and support adequate repletion as a possible prophylaxis for thyroid irAEs.
Keywords: Cancer; Cholecalciferol; Immune checkpoint inhibitors; Immune related adverse events; Immunotherapy; Vitamin D.
© 2023. The Author(s).
Conflict of interest statement
Melissa Bersanelli received funding for the present study from Roche S.p.A. and Seqirus (through FICOG as Institution, no personal fees). She also received, outside the current work: research funding from Pfizer and Novartis (through Institutions); honoraria as a speaker at scientific events (personal fees) by BMS, MSD, IPSEN, Novartis, Astra Zeneca, Pierre Fabre, and Pfizer; as a consultant for advisory role (personal fees) by IPSEN, Novartis, Sanofi, Pierre-Fabre, and Merck; personal fees for copyright transfer by Sciclone Pharmaceuticals, Pierre-Fabre, MSD, IPSEN, Pfizer, and Sanofi. Alessandro Leonetti has received speakers’ fees for Astra-Zeneca and MSD, and has been on advisory boards for BeiGene, Sanofi and Novartis. Alessio Cortellini received grants for consultancies/advisory boards from BMS, MSD, OncoC4, IQVIA, Roche, GSK, AstraZeneca, Access Infinity, Ardelis Health and AlphaSight. He also received speaker fees from AstraZeneca, EISAI, Pierre-Fabre, MSD. Marcello Tiseo received speakers’ and consultants’ fee from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre, Amgen, Merck, Sanofi. He also received institutional research grants from Astra-Zeneca, Boehringer Ingelheim. Sebastiano Buti received honoraria as a speaker at scientific events and in advisory role by BMS, Pfizer; MSD, Ipsen, Roche S.p.A., Eli-Lilly, AstraZeneca, and Novartis; he also received research funding from Novartis. All other authors declared no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

