An oral cholera vaccine in the prevention and/or treatment of inflammatory bowel disease
- PMID: 37639428
- PMCID: PMC10461820
- DOI: 10.1371/journal.pone.0283489
An oral cholera vaccine in the prevention and/or treatment of inflammatory bowel disease
Abstract
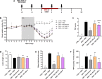
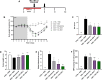
The oral cholera vaccine WC-rBS consists of 4 different inactivated strains of Vibrio cholerae (LPS source) admixed with recombinant cholera toxin B subunit. Because of its unique composition and anti-inflammatory properties reported for both CTB and low doses of LPS from other Gram-negative bacteria, we speculated that WC-rBS might have anti-inflammatory potential in a chronic autoimmune disease such as inflammatory bowel diseases. First in vitro endotoxin tolerance experiments showed the surprising WC-rBS potential in the modulation of inflammatory responses on both PBMCs and THP1 cells. WC-rBS was further evaluated in the Dextran Sodium Sulfate colitis mouse model. Administrated orally at different dosages, WC-rBS vaccine was safe and showed immunomodulatory properties when administered in a preventive mode (before and during the induction of DSS colitis) as well as in a curative mode (after colitis induction); with improvement of disease activity index (from 27 to 73%) and histological score (from 65 to 88%). Interestingly, the highest therapeutic effect of WC-rBS vaccine was observed with the lowest dosage, showing even better anti-inflammatory properties than mesalamine; an approved 5-aminosalicylic acid drug for treating IBD patients. In summary, this is the first time that a prophylactic medicine, safe and approved for prevention of an infectious disease, showed a benefit in an inflammatory bowel disease model, potentially offering a novel therapeutic modality for IBD patients.
Copyright: © 2023 Meunier et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Marine Meunier, Adrian Spillmann, Klaus Schwamborn and Melissa Hanson were all employees of the Valneva group when the work was done. The Swedish subsidiary of the Valneva group owns Dukoral®, i.e. the WC-rBS vaccine. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

