A possible mechanism for the enhanced toxicity of beta-amyloid protofibrils in Alzheimer's disease
- PMID: 37639602
- PMCID: PMC10483626
- DOI: 10.1073/pnas.2309389120
A possible mechanism for the enhanced toxicity of beta-amyloid protofibrils in Alzheimer's disease
Abstract
The amyloid-beta peptide (Aβ) is a driver of Alzheimer's disease (AD). Aβ monomers can aggregate and form larger soluble (oligomers/protofibrils) and insoluble (fibrils) forms. There is evidence that Aβ protofibrils are the most toxic form, but the reasons are not known. Consistent with a critical role for this form of Aβ in AD, a recently FDA-approved therapeutic antibody targeted against protofibrils, lecanemab, slows the progression of AD in patients. The plasma contact system, which can promote coagulation and inflammation, has been implicated in AD pathogenesis. This system is activated by Aβ which could lead to vascular and inflammatory pathologies associated with AD. We show here that the contact system is preferentially activated by protofibrils of Aβ. Aβ protofibrils bind to coagulation factor XII and high molecular weight kininogen and accelerate the activation of the system. Furthermore, lecanemab blocks Aβ protofibril activation of the contact system. This work provides a possible mechanism for Aβ protofibril toxicity in AD and why lecanemab is therapeutically effective.
Keywords: Alzheimer’s; beta-amyloid; coagulation.
Conflict of interest statement
The authors declare no competing interest.
Figures

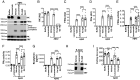
References
-
- Nilsberth C., et al. , The "Arctic" APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat. Neurosci. 4, 887–893 (2001). - PubMed
-
- Tucker S., et al. , The murine version of BAN2401 (mAb158) selectively reduces amyloid-beta protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J. Alzheimer’s Dis. (JAD) 43, 575–588 (2015). - PubMed
-
- van Dyck C. H., et al. , Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

