Exploration of the antibody-drug conjugate clinical landscape
- PMID: 37639687
- PMCID: PMC10464553
- DOI: 10.1080/19420862.2023.2229101
Exploration of the antibody-drug conjugate clinical landscape
Abstract
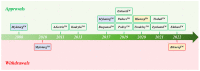
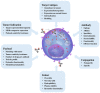
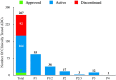
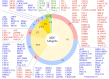
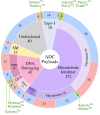
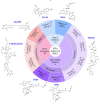
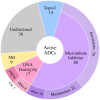
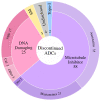
The antibody-drug conjugate (ADC) field has undergone a renaissance, with substantial recent developmental investment and subsequent drug approvals over the past 6 y. In November 2022, ElahereTM became the latest ADC to be approved by the US Food and Drug Administration (FDA). To date, over 260 ADCs have been tested in the clinic against various oncology indications. Here, we review the clinical landscape of ADCs that are currently FDA approved (11), agents currently in clinical trials but not yet approved (164), and candidates discontinued following clinical testing (92). These clinically tested ADCs are further analyzed by their targeting tumor antigen(s), linker, payload choices, and highest clinical stage achieved, highlighting limitations associated with the discontinued drug candidates. Lastly, we discuss biologic engineering modifications preclinically demonstrated to improve the therapeutic index that if incorporated may increase the proportion of molecules that successfully transition to regulatory approval.
Keywords: ADC; Antibody–drug conjugate; clinical trials; drug-to-antibody ratio; linker; payload; site-directed conjugation.
Conflict of interest statement
All authors are employees of Aarvik Therapeutics, Inc. and may have stock and/or stock options or interests in Aarvik Therapeutics, Inc.
Figures


References
-
- Tolcher AW, Carneiro BA, Dowlati A, Razak AR, Chae YK, Villella JA, Coppola S, Englert S, Phillips AC, Souers AJ, et al. A first-in-human study of mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumors: dose escalation results. J Clin Oncol. 2021;39:3015–43. doi:10.1200/JCO.2021.39.15_suppl.3015. - DOI
-
- Sharma M, Carvajal RD, Hanna GJ, Li BT, Moore KN, Pegram MD, Rasco DW, Spira AI, Alonso M, Fang L, et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. J Clin Oncol. 2021;39(15_suppl):2549–2549. doi:10.1200/JCO.2021.39.15_suppl.2549. - DOI
-
- Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, Hinrichs MJ, Bezabeh BZ, Fleming RL, Dimasi N, et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016;29:117–29. doi:10.1016/j.ccell.2015.12.008. PMID: 26766593. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
