Repurposed drug studies on the primary prevention of SARS-CoV-2 infection during the pandemic: systematic review and meta-analysis
- PMID: 37640510
- PMCID: PMC10462970
- DOI: 10.1136/bmjresp-2023-001674
Repurposed drug studies on the primary prevention of SARS-CoV-2 infection during the pandemic: systematic review and meta-analysis
Abstract
Objective: Current evidence on the effectiveness of SARS-CoV-2 prophylaxis is inconclusive. We aimed to systematically evaluate published studies on repurposed drugs for the prevention of laboratory-confirmed SARS-CoV-2 infection and/or COVID-19 among healthy adults.
Design: Systematic review.
Eligibility: Quantitative experimental and observational intervention studies that evaluated the effectiveness of repurposed drugs for the primary prevention of SARS-CoV-2 infection and/or COVID-19 disease.
Data source: PubMed and Embase (1 January 2020-28 September 2022).
Risk of bias: Cochrane Risk of Bias 2.0 and Risk of Bias in Non-Randomised Studies of Interventions tools were applied to assess the quality of studies.
Data analysis: Meta-analyses for each eligible drug were performed if ≥2 similar study designs were available.
Results: In all, 65 (25 trials, 40 observational) and 29 publications were eligible for review and meta-analyses, respectively. Most studies pertained to hydroxychloroquine (32), ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB) (11), statin (8), and ivermectin (8). In trials, hydroxychloroquine prophylaxis reduced laboratory-confirmed SARS-CoV-2 infection (risk ratio: 0.82 (95% CI 0.74 to 0.90), I2=48%), a result largely driven by one clinical trial (weight: 60.5%). Such beneficial effects were not observed in observational studies, nor for prognostic clinical outcomes. Ivermectin did not significantly reduce the risk of SARS-CoV-2 infection (RR: 0.35 (95% CI 0.10 to 1.26), I2=96%) and findings for clinical outcomes were inconsistent. Neither ACEi or ARB were beneficial in reducing SARS-CoV-2 infection. Most of the evidence from clinical trials was of moderate quality and of lower quality in observational studies.
Conclusions: Results from our analysis are insufficient to support an evidence-based repurposed drug policy for SARS-CoV-2 prophylaxis because of inconsistency. In the view of scarce supportive evidence on repurposing drugs for COVID-19, alternative strategies such as immunisation of vulnerable people are warranted to prevent the future waves of infection.
Prospero registration number: CRD42021292797.
Keywords: COVID-19; respiratory infection.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
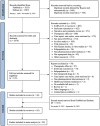




Similar articles
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2021 Jul 28;7(7):CD015017. doi: 10.1002/14651858.CD015017.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD015017. doi: 10.1002/14651858.CD015017.pub3. PMID: 34318930 Free PMC article. Updated.
-
Efficacy of Ivermectin, Chloroquine/Hydroxychloroquine, and Azithromycin in Managing COVID-19: A Systematic Review of Phase III Clinical Trials.Biomedicines. 2024 Sep 27;12(10):2206. doi: 10.3390/biomedicines12102206. Biomedicines. 2024. PMID: 39457519 Free PMC article. Review.
-
Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review.Ann Intern Med. 2020 Aug 4;173(3):195-203. doi: 10.7326/M20-1515. Epub 2020 May 15. Ann Intern Med. 2020. Update in: Ann Intern Med. 2021 Feb;174(2):W25-W29. doi: 10.7326/L20-1446. Update in: Ann Intern Med. 2021 Jun;174(6):W54-W55. doi: 10.7326/L21-0223. PMID: 32422062 Free PMC article. Updated.
-
Pre-exposure prophylaxis with hydroxychloroquine for high-risk healthcare workers during the COVID-19 pandemic: A structured summary of a study protocol for a multicentre, double-blind randomized controlled trial.Trials. 2020 Jul 29;21(1):688. doi: 10.1186/s13063-020-04621-7. Trials. 2020. PMID: 32727613 Free PMC article.
-
PROTECT Trial: A cluster-randomized study with hydroxychloroquine versus observational support for prevention or early-phase treatment of Coronavirus disease (COVID-19): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jul 31;21(1):689. doi: 10.1186/s13063-020-04527-4. Trials. 2020. PMID: 32736597 Free PMC article.
Cited by
-
The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis and clinical assessment: an updated meta-analysis of randomized trials.J Thorac Dis. 2024 May 31;16(5):2983-2993. doi: 10.21037/jtd-23-1043. Epub 2024 May 29. J Thorac Dis. 2024. PMID: 38883686 Free PMC article.
-
Antimalarial compounds exhibit variant- and cell-type-specific activity against SARS-CoV-2 isolated in Panama.Front Pharmacol. 2025 Jun 4;16:1537053. doi: 10.3389/fphar.2025.1537053. eCollection 2025. Front Pharmacol. 2025. PMID: 40535766 Free PMC article.
-
A Transformer-Based Framework for Counterfactual Estimation of Antihypertensive Treatment Effect on COVID-19 Infection Risk - A Proof-of-Concept Study.Am J Hypertens. 2025 Jul 15;38(8):595-604. doi: 10.1093/ajh/hpaf055. Am J Hypertens. 2025. PMID: 40247607 Free PMC article.
-
Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection and severe outcomes in adults: a systematic review and meta-analysis of European studies published up to 22 January 2024.Eur Respir Rev. 2025 Feb 19;34(175):240222. doi: 10.1183/16000617.0222-2024. Print 2025 Jan. Eur Respir Rev. 2025. PMID: 39971395 Free PMC article.
References
-
- Johns Hopkins University CSSE COVID-19 data. Available: https://ourworldindata.org/explorers/coronavirus-data-explorer
-
- World Health Organization . Statement on the fifteenth meeting of the IHR (2005) emergency Committee on the COVID-19 pandemic. 2023. Available: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
