A cost-effectiveness analysis of avelumab plus best supportive care versus best supportive care alone as first-line maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma in Taiwan
- PMID: 37640556
- PMCID: PMC10598249
- DOI: 10.1002/cnr2.1887
A cost-effectiveness analysis of avelumab plus best supportive care versus best supportive care alone as first-line maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma in Taiwan
Abstract
Background: Patients with locally advanced or metastatic urothelial carcinoma have limited treatment options and a poor prognosis. The JAVELIN Bladder 100 trial showed that avelumab as first-line maintenance plus best supportive care significantly prolonged overall survival and progression-free survival versus best supportive care alone in patients with locally advanced or metastatic urothelial carcinoma that had not progressed with first-line platinum-containing chemotherapy.
Aims: We assessed whether avelumab plus best supportive care is a cost-effective treatment option versus best supportive care alone in this patient group in Taiwan.
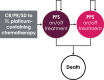
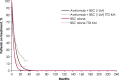
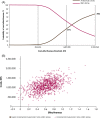
Methods and results: A partitioned survival model was used to estimate the costs and effects of avelumab plus best supportive care versus best supportive care alone over a 20-year time horizon from the perspective of Taiwan's National Health Insurance Administration. Patient-level data from JAVELIN Bladder 100 on efficacy, safety, utility, and time on treatment were analyzed to provide parameters for the model. Log-normal and Weibull distributions were used for overall survival and progression-free survival, respectively. Costs of healthcare resources, drug acquisition, adverse events, and progression were identified through publicly available data sources and clinician interviews. The model estimated total costs, life years, and quality-adjusted life years. In the modeled base case, avelumab plus best supportive care increased survival versus best supportive care alone by 0.79 life years (2.93 vs. 2.14) and 0.61 quality-adjusted life years (2.15 vs. 1.54). The incremental cost-effectiveness ratio for avelumab plus best supportive care versus best supportive care alone was NT$1 827 680. Most (78%) of the probabilistic sensitivity analyses fell below three times the gross domestic product per capita. Scenario analysis indicated that life year and quality-adjusted life year gains were most sensitive to alternative survival extrapolations for both avelumab plus best supportive care and best supportive care alone.
Conclusion: Avelumab first-line maintenance therapy combined with best supportive care was determined as a cost-effective treatment strategy for patients in Taiwan diagnosed with locally advanced or metastatic urothelial carcinoma that had not progressed with platinum-containing chemotherapy.
Keywords: JAVELIN Bladder 100 study; Taiwan; avelumab; economic model; health technology assessment; urothelial carcinoma.
© 2023 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
Po‐Jung Su has nothing to disclose. Venediktos Kapetanakis is an employee of Evidera, London, UK. Ying Xiao was an employee of Evidera, London, UK at the time the analysis was conducted. Amy Y. Lin was an employee of Merck Ltd., Taipei, Taiwan, an affiliate of Merck KGaA, Darmstadt, Germany, at the time the analysis was conducted. Connie Goh is an employee of Merck Ltd., Taipei, Taiwan, an affiliate of Merck KGaA, Darmstadt, Germany. Ethan Wu and Kevin Liu are employees of Pfizer, Taipei, Taiwan. Patrick Chou and Kaitlin Kuo are employees of IQVIA Solutions Taiwan Ltd., Taipei, Taiwan. Roberto Palencia was an employee of the healthcare business of Merck KGaA, Darmstadt, Germany at the time the analysis was conducted. Mairead Kearney is an employee of the healthcare business of Merck KGaA, Darmstadt, Germany. Jane Chang is an employee of Pfizer, New York, NY, USA. Agnes Benedict is an employee of Evidera, Budapest, Hungary.
Figures

References
-
- Health Promotion Administration . Ministry of Health and Welfare Taiwan. Cancer Registry Annual Report December 2020. https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=13498. Accessed May 22, 2023
-
- Taiwan Urological Oncology Association . Urology Cancer Management Guideline. (Aug. 23, 2021). http://www.tua.org.tw/tua/tw/latest-news/announcement/1155-tua2020-2020-.... Accessed May, 22 2023
-
- NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer. V2.2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

