Tricolor visible wavelength-selective photodegradable hydrogel biomaterials
- PMID: 37640707
- PMCID: PMC10462736
- DOI: 10.1038/s41467-023-40805-w
Tricolor visible wavelength-selective photodegradable hydrogel biomaterials
Abstract
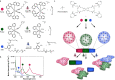
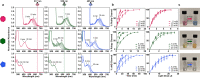
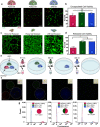
Photodynamic hydrogel biomaterials have demonstrated great potential for user-triggered therapeutic release, patterned organoid development, and four-dimensional control over advanced cell fates in vitro. Current photosensitive materials are constrained by their reliance on high-energy ultraviolet light (<400 nm) that offers poor tissue penetrance and limits access to the broader visible spectrum. Here, we report a family of three photolabile material crosslinkers that respond rapidly and with unique tricolor wavelength-selectivity to low-energy visible light (400-617 nm). We show that when mixed with multifunctional poly(ethylene glycol) macromolecular precursors, ruthenium polypyridyl- and ortho-nitrobenzyl (oNB)-based crosslinkers yield cytocompatible biomaterials that can undergo spatiotemporally patterned, uniform bulk softening, and multiplexed degradation several centimeters deep through complex tissue. We demonstrate that encapsulated living cells within these photoresponsive gels show high viability and can be successfully recovered from the hydrogels following photodegradation. Moving forward, we anticipate that these advanced material platforms will enable new studies in 3D mechanobiology, controlled drug delivery, and next-generation tissue engineering applications.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ruskowitz ER, DeForest CA. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat. Rev. Mater. 2018;3:17087.
-
- Rapp TL, DeForest CA. Visible Light‐Responsive Dynamic Biomaterials: Going Deeper and Triggering More. Adv. Healthc. Mater. 2020;9:1901553. - PubMed
-
- Munoz-Robles BG, Kopyeva I, DeForest CA. Biomaterials: Surface Patterning of Hydrogel Biomaterials to Probe and Direct Cell–Matrix Interactions (Adv. Mater. Interfaces 21/2020) Adv. Mater. Interf. 2020;7:2070116.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

