Knockdown of Porf-2 restores visual function after optic nerve crush injury
- PMID: 37640747
- PMCID: PMC10462692
- DOI: 10.1038/s41419-023-06087-2
Knockdown of Porf-2 restores visual function after optic nerve crush injury
Abstract
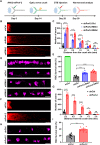
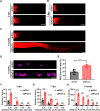
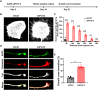
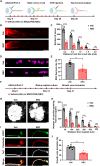
Retinal ganglion cells (RGCs), the sole output neurons in the eyes, are vulnerable to diverse insults in many pathological conditions, which can lead to permanent vision dysfunction. However, the molecular and cellular mechanisms that contribute to protecting RGCs and their axons from injuries are not completely known. Here, we identify that Porf-2, a member of the Rho GTPase activating protein gene group, is upregulated in RGCs after optic nerve crush. Knockdown of Porf-2 protects RGCs from apoptosis and promotes long-distance optic nerve regeneration after crush injury in both young and aged mice in vivo. In vitro, we find that inhibition of Porf-2 induces axon growth and growth cone formation in retinal explants. Inhibition of Porf-2 provides long-term and post-injury protection to RGCs and eventually promotes the recovery of visual function after crush injury in mice. These findings reveal a neuroprotective impact of the inhibition of Porf-2 on RGC survival and axon regeneration after optic nerve injury, providing a potential therapeutic strategy for vision restoration in patients with traumatic optic neuropathy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
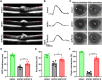
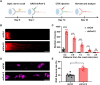
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

