Natriuresis-guided diuretic therapy in acute heart failure: a pragmatic randomized trial
- PMID: 37640861
- PMCID: PMC10579092
- DOI: 10.1038/s41591-023-02532-z
Natriuresis-guided diuretic therapy in acute heart failure: a pragmatic randomized trial
Abstract
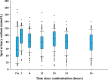
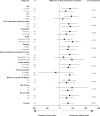
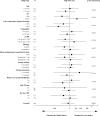
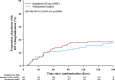
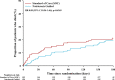
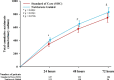
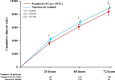
Measurement of natriuresis has been suggested as a reliable, easily obtainable biomarker for assessment of the response to diuretic treatment in patients with acute heart failure (AHF). Here, to assess whether natriuresis-guided diuretic therapy in patients with AHF improves natriuresis and clinical outcomes, we conducted the pragmatic, open-label Pragmatic Urinary Sodium-based algoritHm in Acute Heart Failure trial, in which 310 patients (45% female) with AHF requiring treatment with intravenous loop diuretics were randomly assigned to natriuresis-guided therapy or standard of care (SOC). In the natriuresis-guided arm, natriuresis was determined at set timepoints, prompting treatment intensification if spot urinary sodium levels were <70 mmol l-1. The dual primary endpoints were 24 h urinary sodium excretion and a combined endpoint of time to all-cause mortality or adjudicated heart failure rehospitalization at 180 days. The first primary endpoint was met, as natriuresis in the natriuresis-guided and SOC arms was 409 ± 178 mmol arm versus 345 ± 202 mmol, respectively (P = 0.0061). However, there were no significant differences between the two arms for the combined endpoint of time to all-cause mortality or first heart failure rehospitalization, which occurred in 46 (31%) and 50 (31%) of patients in the natriuresis-guided and SOC arms, respectively (hazard ratio 0.92 [95% confidence interval 0.62-1.38], P = 0.6980). These findings suggest that natriuresis-guided therapy could be a first step towards personalized treatment of AHF. ClinicalTrials.gov registration: NCT04606927 .
© 2023. The Author(s).
Conflict of interest statement
J.M.t.M. is supported by a Dekker grant of the Dutch Heart Foundation (2020T012) for this study. J.M.t.M. declares speaker fees to her institution from Boehringer Ingelheim and Novartis. P.v.d.M. declares speaker and consultancy fees to his institution from Vifor Pharma, Novo Nordisk, Pfizer, AstraZeneca, Ionis and Pharma Nord. J.E.C. declares speaker fees from Novartis, and has received fees from Cardiolysis for participation on a DSMB or advisory board. A.A.V. has received research support and/or has been a consultant for AnaCardio, Bayer, BMI, Boehringer Ingelheim, Corteria, Cytokinetics, Eli Lily, Merck, Novartis, Novo Nordisk and Roche Diagnostics. K.D. declares speaker and consultancy fees to his institution from Boehringer Ingelheim, AstraZeneca, Abbott, FIRE1 and EchoSense. The other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

