Sirtuin 6 protects against podocyte injury by blocking the renin-angiotensin system by inhibiting the Wnt1/β-catenin pathway
- PMID: 37640899
- PMCID: PMC10770168
- DOI: 10.1038/s41401-023-01148-w
Sirtuin 6 protects against podocyte injury by blocking the renin-angiotensin system by inhibiting the Wnt1/β-catenin pathway
Abstract
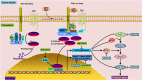
Sirtuins (Sirts) are a family of nicotinamide adenine dinucleotide-dependent protein deacetylases that share diverse cellular functions. Increasing evidence shows that Sirts play a critical role in podocyte injury, which is a major determinant of proteinuria-associated renal disease. Membranous nephropathy (MN) is a typical glomerular disease in which podocyte damage mediates proteinuria development. In this study we investigated the molecular mechanisms underlying the regulatory roles of Sirt in podocyte injury in MN patients, rats with cationic bovine serum albumin (CBSA)-induced MN and zymosan activation serum (ZAS)-stimulated podocytes. Compared with healthy controls, MN patients showed significant reduction in intrarenal Sirt1 and Sirt6 protein expression. In CBSA-induced MN rats, significant reduction in intrarenal Sirt1, Sirt3 and Sirt6 protein expression was observed. However, only significant decrease in Sirt6 protein expression was found in ZAS-stimulated podocytes. MN patients showed significantly upregulated protein expression of Wnt1 and β-catenin and renin-angiotensin system (RAS) components in glomeruli. CBSA-induced MN rats exhibited significantly upregulated protein expression of intrarenal Wnt1 and β-catenin and their downstream gene products as well as RAS components. Similar results were observed in ZAS-stimulated podocytes. In ZAS-stimulated podocytes, treatment with a specific Sirt6 activator UBCS039 preserved the protein expression of podocin, nephrin and podocalyxin, accompanied by significant inhibition of the protein expression of β-catenin and its downstream gene products, including Snail1 and Twist; treatment with a β-catenin inhibitor ICG-001 significantly preserved the expression of podocyte-specific proteins and inhibited the upregulation of downstream β-catenin gene products accompanied by significant suppression of the protein expression of RAS components. Thus, we demonstrate that Sirt6 ameliorates podocyte injury by blocking RAS signalling via the Wnt1/β-catenin pathway. Sirt6 is a specific therapeutic target for the treatment of podocyte damage-associated renal disease.
Keywords: Sirtuins; UBCS039; Wnt1/β-catenin pathway; membranous nephropathy; podocyte injury; renin-angiotensin system.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

