Programmable (proSA®) vs. fixed (SHUNTASSISTANT®) gravitational valves in pediatric patients with hydrocephalus: a 16-year retrospective single-center comparative study with biomechanical analysis
- PMID: 37640980
- PMCID: PMC10739459
- DOI: 10.1007/s00701-023-05751-y
Programmable (proSA®) vs. fixed (SHUNTASSISTANT®) gravitational valves in pediatric patients with hydrocephalus: a 16-year retrospective single-center comparative study with biomechanical analysis
Abstract
Purpose: In pediatric hydrocephalus (HC) treatment, programmable gravitational valves offer greater flexibility to manage overdrainage during children's growth. However, it remains unclear whether these devices provide better outcomes rather than their precursors. The study assessed the benefit from programmability of gravitational valve, i.e., programmable-SHUNTASSISTANT (proSA®) vs. SHUNTASSISTANT® (SA®).
Methods: Clinical records and imaging of pediatric patients with hydrocephalus of non-tumoral etiology treated with fixed (SA®) or programmable (proSA®) gravitational valves between January 2006 and January 2022 were analyzed in a retrospective single-center study. Valve survival was compared in relation to age and etiology. Lately explanted valves received biomechanical analysis.
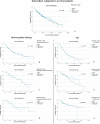
Results: A total of 391 gravitational valves (254 SA® and 137 proSA®) were inserted in 244 patients (n = 134 males). One hundred thirty-three SA® (52.4%) and 67 proSA® (48.9%) were explanted during a follow-up of 81.1 ± 46.3 months. Valve survival rate at 1 and 5 years with proSA® was 87.6% and 60.6% compared to 81.9% and 58.7% with SA®, with mean survival time 56.4 ± 35.01 and 51.4 ± 43.0 months, respectively (P = 0.245). Age < 2 years at implantation correlated with significantly lower valve survival rates (P < 0.001), while HC etiology showed no significant impact. Overdrainage alone accounted for more SA® revisions (39.8% vs. 3.1%, P < 0.001), while dysfunctions of the adjustment system represented the first cause of valve replacement in proSA® cohort (45.3%). The biomechanical analysis performed on 41 proSA® and 31 SA® showed deposits on the valve's internal surface in 97.6% and 90.3% of cases.
Conclusion: Our comparative study between proSA® and SA® valves in pediatric HC demonstrated that both valves showed similar survival rates, regardless of etiology but only with young age at implantation. The programmability may be beneficial in preventing sequelae of chronic overdrainage but does not reduce need for valve revision and proSA® valve should be considered in selected cases in growing children older than 2 years.
Keywords: Gravitational valve; Hydrocephalus; Overdrainage; SHUNTASSISTANT®; Underdrainage; proSA®.
© 2023. The Author(s).
Conflict of interest statement
August von Hardenberg and Christoph Miethke work at Miethke GmbH & Co. KG and supervised the valve analysis. The authors declare no conflict of interest.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

