High efficacy of chlorfenapyr-based net Interceptor® G2 against pyrethroid-resistant malaria vectors from Cameroon
- PMID: 37641108
- PMCID: PMC10463949
- DOI: 10.1186/s40249-023-01132-w
High efficacy of chlorfenapyr-based net Interceptor® G2 against pyrethroid-resistant malaria vectors from Cameroon
Abstract
Background: The increasing reports of resistance to pyrethroid insecticides associated with reduced efficacy of pyrethroid-only interventions highlight the urgency of introducing new non-pyrethroid-only control tools. Here, we investigated the performance of piperonyl-butoxide (PBO)-pyrethroid [Permanet 3.0 (P3.0)] and dual active ingredients (AI) nets [Interceptor G2 (IG2): containing pyrethroids and chlorfenapyr and Royal Guard (RG): containing pyrethroids and pyriproxyfen] compared to pyrethroid-only net Royal Sentry (RS) against pyrethroid-resistant malaria vectors in Cameroon.
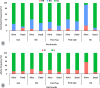
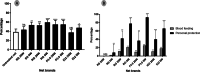
Methods: The efficacy of these tools was firstly evaluated on Anopheles gambiae s.l. and Anopheles funestus s.l. from Gounougou, Mibellon, Mangoum, Nkolondom, and Elende using cone/tunnel assays. In addition, experimental hut trials (EHT) were performed to evaluate the performance of unwashed and 20 times washed nets in semi-field conditions. Furthermore, pyrethroid-resistant markers were genotyped in dead vs alive, blood-fed vs unfed mosquitoes after exposure to the nets to evaluate the impact of these markers on net performance. The XLSTAT software was used to calculate the various entomological outcomes and the Chi-square test was used to compare the efficacy of various nets. The odds ratio and Fisher exact test were then used to establish the statistical significance of any association between insecticide resistance markers and bed net efficacy.
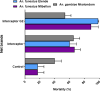
Results: Interceptor G2 was the most effective net against wild pyrethroid-resistant An. funestus followed by Permanet 3.0. In EHT, this net induced up to 87.8% mortality [95% confidence interval (CI): 83.5-92.1%) and 55.6% (95% CI: 48.5-62.7%) after 20 washes whilst unwashed pyrethroid-only net (Royal Sentry) killed just 18.2% (95% CI: 13.4-22.9%) of host-seeking An. funestus. The unwashed Permanet 3.0 killed up to 53.8% (95% CI: 44.3-63.4%) of field-resistant mosquitoes and 47.2% (95% CI: 37.7-56.7%) when washed 20 times, and the Royal Guard 13.2% (95% CI: 9.0-17.3%) for unwashed net and 8.5% (95% CI: 5.7-11.4%) for the 20 washed net. Interceptor G2, Permanet 3.0, and Royal Guard provided better personal protection (blood-feeding inhibition 66.2%, 77.8%, and 92.8%, respectively) compared to pyrethroid-only net Royal Sentry (8.4%). Interestingly, a negative association was found between kdrw and the chlorfenapyr-based net Interceptor G2 (χ2 = 138; P < 0.0001) with homozygote-resistant mosquitoes predominantly found in the dead ones.
Conclusions: The high mortality recorded with Interceptor G2 against pyrethroid-resistant malaria vectors in this study provides first semi-field evidence of high efficacy against these major malaria vectors in Cameroon encouraging the implementation of this novel net for malaria control in the country. However, the performance of this net should be established in other locations and on other major malaria vectors before implementation at a large scale.
Keywords: Anopheles; Dual active ingredient nets; Insecticide resistance; Interceptor G2; Malaria.
© 2023. National Institute of Parasitic Diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Efficacy of Interceptor G2, Royal Guard and PermaNet 3.0 against pyrethroid-resistant Anopheles gambiae s.l. from Za-Kpota, southern Benin: an experimental hut trial.Parasit Vectors. 2024 Jul 11;17(1):300. doi: 10.1186/s13071-024-06372-9. Parasit Vectors. 2024. PMID: 38992693 Free PMC article.
-
Efficacy of interceptor® G2, a long-lasting insecticide mixture net treated with chlorfenapyr and alpha-cypermethrin against Anopheles funestus: experimental hut trials in north-eastern Tanzania.Malar J. 2021 Apr 9;20(1):180. doi: 10.1186/s12936-021-03716-z. Malar J. 2021. PMID: 33836778 Free PMC article. Clinical Trial.
-
Does washing insecticide-treated nets 20 times for experimental hut evaluations provide a suitable proxy for their end-of-life performance under household conditions?Parasit Vectors. 2025 Apr 21;18(1):148. doi: 10.1186/s13071-025-06743-w. Parasit Vectors. 2025. PMID: 40259380 Free PMC article.
-
Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa.Cochrane Database Syst Rev. 2018 Nov 29;11(11):CD012776. doi: 10.1002/14651858.CD012776.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 May 24;5:CD012776. doi: 10.1002/14651858.CD012776.pub3. PMID: 30488945 Free PMC article. Updated.
-
Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa.Cochrane Database Syst Rev. 2021 May 24;5(5):CD012776. doi: 10.1002/14651858.CD012776.pub3. Cochrane Database Syst Rev. 2021. PMID: 34027998 Free PMC article.
Cited by
-
Substrate promiscuity of key resistance P450s confers clothianidin resistance while increasing chlorfenapyr potency in malaria vectors.Cell Rep. 2024 Aug 27;43(8):114566. doi: 10.1016/j.celrep.2024.114566. Epub 2024 Jul 31. Cell Rep. 2024. PMID: 39088320 Free PMC article.
-
Temporal evolution of insecticide resistance and bionomics in Anopheles funestus, a key malaria vector in Uganda.Sci Rep. 2024 Dec 30;14(1):32027. doi: 10.1038/s41598-024-83689-6. Sci Rep. 2024. PMID: 39738472 Free PMC article.
-
Evaluation of bio-efficacy of field-aged novel long-lasting insecticidal nets (PBO, chlorfenapyr or pyriproxyfen combined with pyrethroid) against Anopheles gambiae (s.s.) in Tanzania.Curr Res Parasitol Vector Borne Dis. 2024 Sep 23;6:100216. doi: 10.1016/j.crpvbd.2024.100216. eCollection 2024. Curr Res Parasitol Vector Borne Dis. 2024. PMID: 39399651 Free PMC article.
-
Longitudinal survey of insecticide resistance in a village of central region of Burkina Faso reveals co-occurrence of 1014F, 1014S and 402L mutations in Anopheles coluzzii and Anopheles arabiensis.Malar J. 2024 Aug 20;23(1):250. doi: 10.1186/s12936-024-05069-9. Malar J. 2024. PMID: 39164725 Free PMC article.
-
Efficacy of Interceptor G2, Royal Guard and PermaNet 3.0 against pyrethroid-resistant Anopheles gambiae s.l. from Za-Kpota, southern Benin: an experimental hut trial.Parasit Vectors. 2024 Jul 11;17(1):300. doi: 10.1186/s13071-024-06372-9. Parasit Vectors. 2024. PMID: 38992693 Free PMC article.
References
-
- Koudou B, Tano Y, Doumbia M, Nsanzabana C, Cisse G, Girardin O, Dao D, N'Goran E, Vounatsou P, Bordmann G. Insecticide-treated bed nets and curtains for preventing malaria. Med Vet Entomol. 2005;19(1):27–37. - PubMed
-
- Protopopoff N, Wright A, West PA, Tigererwa R, Mosha FW, Kisinza W, Kleinschmidt I, Rowland M. Combination of insecticide treated nets and indoor residual spraying in northern Tanzania provides additional reduction in vector population density and malaria transmission rates compared to insecticide treated nets alone: a randomised control trial. PLoS ONE. 2015;10(11):e0142671. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

