A neuropathologic feature of brain aging: multi-lumen vascular profiles
- PMID: 37641147
- PMCID: PMC10464008
- DOI: 10.1186/s40478-023-01638-2
A neuropathologic feature of brain aging: multi-lumen vascular profiles
Abstract
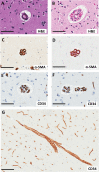
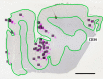
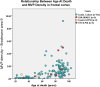
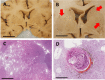
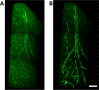
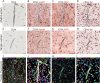
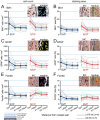
Cerebrovascular pathologies other than frank infarctions are commonly seen in aged brains. Here, we focus on multi-lumen vascular profiles (MVPs), which are characterized by multiple vessel lumens enclosed in a single vascular channel. Little information exists on the prevalence, risk factors, and co-pathologies of MVPs. Therefore, we used samples and data from the University of Kentucky Alzheimer's Disease Research Center (n = 91), the University of Kentucky Pathology Department (n = 31), and the University of Pittsburgh Pathology Department (n = 4) to study MVPs. Age at death was correlated with MVP density in the frontal neocortex, Brodmann Area 9 (r = 0.51; p < 0.0001). Exploratory analyses were performed to evaluate the association between conventional vascular risk factors (e.g., hypertension, diabetes), cardiovascular diseases (e.g., heart attack, arrhythmia), and cerebrovascular disease (e.g., stroke); the only nominal association with MVP density was a self-reported history of brain trauma (Prevalence Ratio = 2.1; 95 CI 1.1-3.9, before correcting for multiple comparisons). No specific associations were detected between neuropathological (e.g., brain arteriolosclerosis) or genetic (e.g., APOE) variables and MVP density. Using a tissue clearing method called SeeDB, we provide 3-dimensional images of MVPs in brain tissue. We conclude that MVPs are an age-related brain pathology and more work is required to identify their clinical-pathological correlation and associated risk factors.
Keywords: Endothelial; SVD; Senescence; Small vessel disease; TBI; VCID; VTE.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Genetic susceptibility for ischemic infarction and arteriolosclerosis based on neuropathologic evaluations.Cerebrovasc Dis. 2013;36(3):181-188. doi: 10.1159/000352054. Epub 2013 Oct 12. Cerebrovasc Dis. 2013. PMID: 24135527 Free PMC article.
-
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons.Brain Pathol. 2021 May;31(3):e12939. doi: 10.1111/bpa.12939. Epub 2021 Feb 23. Brain Pathol. 2021. PMID: 33624322 Free PMC article.
-
Brain pathologies in extreme old age.Neurobiol Aging. 2016 Jan;37:1-11. doi: 10.1016/j.neurobiolaging.2015.10.009. Epub 2015 Oct 19. Neurobiol Aging. 2016. PMID: 26597697 Free PMC article.
-
Disordered APP metabolism and neurovasculature in trauma and aging: Combined risks for chronic neurodegenerative disorders.Ageing Res Rev. 2017 Mar;34:51-63. doi: 10.1016/j.arr.2016.11.003. Epub 2016 Nov 6. Ageing Res Rev. 2017. PMID: 27829172 Free PMC article. Review.
-
Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE).Metabolism. 2019 Nov;100S:153943. doi: 10.1016/j.metabol.2019.07.007. Metabolism. 2019. PMID: 31610856 Review.
Cited by
-
Increased frontocortical microvascular raspberry density in frontotemporal lobar degeneration compared to Lewy body disease and control cases: a neuropathological study.Free Neuropathol. 2025 Mar 4;6:7. doi: 10.17879/freeneuropathology-2025-6178. eCollection 2025 Jan. Free Neuropathol. 2025. PMID: 40052111 Free PMC article.
-
Cortical microvascular raspberries and ageing: an independent but not exclusive relationship.Acta Neuropathol Commun. 2023 Dec 12;11(1):195. doi: 10.1186/s40478-023-01700-z. Acta Neuropathol Commun. 2023. PMID: 38087325 Free PMC article.
References
-
- Polvikoski TM, van Straaten EC, Barkhof F, Sulkava R, Aronen HJ, Niinisto L, Oinas M, Scheltens P, Erkinjuntti T, Kalaria RN. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–2078. doi: 10.1212/WNL.0b013e318200d6f9. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

