Longitudinal changes of SARA scale in Friedreich ataxia: Strong influence of baseline score and age at onset
- PMID: 37641437
- PMCID: PMC10647003
- DOI: 10.1002/acn3.51886
Longitudinal changes of SARA scale in Friedreich ataxia: Strong influence of baseline score and age at onset
Abstract
Background: The Scale for Assessment and Rating of Ataxia (SARA) is widely used in different types of ataxias and has been chosen as the primary outcome measure in the European natural history study for Friedreich ataxia (FA).
Methods: To assess distribution and longitudinal changes of SARA scores and its single items, we analyzed SARA scores of 502 patients with typical-onset FA (<25 years) participating in the 4-year prospective European FA Consortium for Translational Studies (EFACTS). Pattern of disease progression was determined using linear mixed-effects regression models. The chosen statistical model was re-fitted in order to estimate parameters and predict disease progression. Median time-to-change and rate of score progression were estimated using the Kaplan-Meier method and weighted linear regression models, respectively.
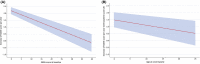
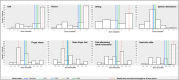
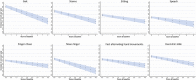
Results: SARA score at study enrollment and age at onset were the major predictive factors of total score progression during the 4-year follow-up. To a less extent, age at evaluation also influenced the speed of SARA progression, while disease duration did not improve the prediction of the statistical model. Temporal dynamics of total SARA and items showed a great variability in the speed of score increase during disease progression. Gait item had the highest annual progression rate, with median time for one-point score increase of 1 to 2 years.
Interpretation: Analyses of statistical properties of SARA suggest a variable sensitivity of the scale at different disease stages, and provide important information for population selection and result interpretation in future clinical trials.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Mariotti C is a site principal investigator for clinical trials sponsored by F. Hoffmann‐La Roche Ltd; Reata Pharmaceuticals, and Prilenia Therapeutics, and received consultancy fees for advisory board from Reata Swiss International. Schöls L is receiving research support from the European Commission (EU), the German Research Fundation (DFG), the Bundesministerium für Bildung und Forschung (BMBF), the Bundesministerium für Gesundheit (BMG), and Servier. He is principal investigator for clinical trials sponsored by PTC Therapeutics and Stealth BioTherapeutics. Within the last 24 months, he received consulting fees from Vico Therapeutics, Lilly and Reata Swiss International. Klockgether T is receiving research support from the Bundesministerium für Bildung und Forschung (BMBF), the National Institutes of Health (NIH), and Servier. Within the last 24 months, he has received consulting fees from Biogen, UCB, and Vico Therapeutics. Reetz K has received grants from the German Federal Ministry of Education and Research (BMBF 01GQ1402, 01DN18022), the German Research Foundation (IRTG 2150, ZUK32/1), Alzheimer Forschung Initiative e.V. (AFI 13812, NL‐18002CB), and honoraria for presentations or advisory boards from Biogen and Roche as well as clinical trial grants from Pfizer, Merck, Minoryx, Biogen, and Roche. Jörg B. Schulz has received consultancy fees and Speaker honoraria by Reata Swiss International, Biogen, Eisai, Lilly, Roche, and Novartis. Boesch S is a site principal investigator for clinical trials sponsored by Reata Pharmaceuticals, and received consultancy fees for advisory board from VICO and Reata Swiss International.
Figures

References
-
- Schmitz‐Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717‐1720. - PubMed
-
- Jacobi H, Tezenas du Montcel S, Bauer P, et al. Long‐term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14(11):1101‐11083. - PubMed
-
- Dürr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996;335(16):1169‐1175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

