First-line nivolumab, paclitaxel, carboplatin, and bevacizumab for advanced non-squamous non-small cell lung cancer: Updated survival analysis of the ONO-4538-52/TASUKI-52 randomized controlled trial
- PMID: 37641544
- PMCID: PMC10501244
- DOI: 10.1002/cam4.6348
First-line nivolumab, paclitaxel, carboplatin, and bevacizumab for advanced non-squamous non-small cell lung cancer: Updated survival analysis of the ONO-4538-52/TASUKI-52 randomized controlled trial
Abstract
Background: ONO-4538-52/TASUKI-52 was performed in Japan, Korea, and Taiwan to determine the oncological effectiveness and safety of combining nivolumab or placebo with bevacizumab plus platinum chemotherapy for the initial (first-line) treatment of patients with advanced non-squamous non-small cell lung cancer (nsNSCLC). At the interim analysis (minimum follow-up, 7.4 months), the independent radiology review committee-assessed progression-free survival was significantly longer in the nivolumab arm, but overall survival (OS) data were immature.
Methods: Here, we present the updated OS data. Patients with treatment-naïve stage IIIB/IV or recurrent nsNSCLC without driver mutations in ALK, EGFR, or ROS1, were randomized 1:1 to receive either nivolumab or placebo. Patients in both arms received paclitaxel, carboplatin, and bevacizumab, administered 3-weekly for a maximum of 6 cycles. Nivolumab/placebo and bevacizumab were subsequently continued until disease progression or unacceptable toxicity.
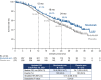
Results: Overall, 550 patients were randomized. At the time of the analysis (minimum follow-up: 19.4 months), the median OS was longer in the nivolumab arm than in the placebo arm (30.8 vs. 24.7 months; hazard ratio 0.74, 95% confidence interval 0.58-0.94). The 12-month OS rates were 81.3% vs. 76.3% in the nivolumab vs. placebo arms, respectively. The respective 18-month OS rates were 69.0% vs. 61.9%.
Conclusion: Nivolumab plus platinum chemotherapy and bevacizumab demonstrated longer OS vs. the placebo combination. We believe this regimen is viable as a standard, first-line treatment for patients with advanced nsNSCLC without driver mutations in ALK, EGFR, or ROS1.
Trial registration: ClinicalTrials.gov NCT03117049.
Keywords: bevacizumab; chemotherapy; nivolumab; non-squamous non-small cell lung cancer; survival.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb funded the study, provided the study drugs, and were involved in the collection, analysis, and interpretation of the data. Employees of the sponsors reviewed and commented on the manuscript. The authors had full access to the data and took final responsibility for the decision to publish the manuscript.
The authors report the following disclosures; remuneration of 1 million yen or more: S.T. (Ono Pharmaceutical); lecture fees/honoraria/other fees of 500,000 yen or more: H.‐R.K. (Ono Pharmaceutical, AstraZeneca, Roche, Boehringer Ingelheim), S.S. (Ono Pharmaceutical, Bristol‐Myers Squibb), T.H. (Ono Pharmaceutical, Bristol‐Myers Squibb), K.‐H.L. (Bristol‐Myers Squibb, MSD, AstraZeneca, Pfizer, and Eli Lilly), T.Y. (Ono Pharmaceutical, Chugai, AstraZeneca), H.T. (AstraZeneca, Chugai), C.‐T.Y. (Ono Pharmaceutical, Boehringer Ingelheim, AstraZeneca, Chugai, Pfizer, Takeda, Novartis, Roche, Eli Lilly, Merck, MSD), M.N. (Ono Pharmaceutical, Bristol‐Myers Squibb), Y.O. (AstraZeneca, Chugai), T.T. (Cmic ShiftZero), H.A. (AstraZeneca), N.Y. (MSD, AstraZeneca, Chugai, Eli Lilly, Boehringer‐Ingelheim, Pfizer, Ono Pharmaceutical, Takeda), K.N. (Eli Lilly, Chugai, Ono Pharmaceutical); annual contract research grants of 1 million yen or more: H.‐R.K (Ono Pharmaceutical), S.S. (Ono Pharmaceutical, Bristol‐Myers Squibb), J.‐S.L. (Ono Pharmaceutical), J.‐H.K. (Ono Pharmaceutical), N.I. (Ono Pharmaceutical), T.H. (Ono Pharmaceutical, Bristol‐Myers Squibb), K.‐H.L. (Ono Pharmaceutical), T.Y. (Ono Pharmaceutical, Chugai, AstraZeneca, Amgen, Novartis, Bristol‐Myers Squibb, Takeda), H.T. (Ono Pharmaceutical), C.‐T.Y. (Ono Pharmaceutical), M.N. (Ono Pharmaceutical, Bristol‐Myers Squibb), Y.O. (AstraZeneca, Chugai, Eli Lilly, Ono Pharmaceutical, Bristol‐Myers Squibb, Kirin, Dainippon‐Sumitomo, Pfizer, Taiho, Novartis, Takeda, Kissei, Daiichi‐Sankyo, Janssen, LOXO), N.Y. (Chugai, Toppan printing, Cmic ShiftZero, MSD, Pfizer, PPD, IQVIA, Eli Lilly, A2 healthcare, Boehringer Ingelheim, Terumo, Takeda, AstraZeneca, Amgen, Jansen, Taiho, Ono Pharmaceutical), C.‐J.Y. (Ono Pharmaceutical), H.A. (Chugai, Amgen), K.N. (Ono Pharmaceutical, MSD, Daiichi Sankyo, Taiho, Chugai, SYNEOS HEALTH CLINICAL, Japan Clinical Research Operations, AstraZeneca, IQVIA Services JAPAN, Covance Japan, Takeda, GlaxoSmithKline, Sanofi, EPS Corporation, Novartis, Medical Research Support); scholarship/endowments of 1 million yen or more: N.Y. (Taiho, Chugai, Daiichi‐Sankyo, Ono Pharmaceutical), K.N. (Takeda, Chugai, Ono Pharmaceutical). Y.O. is a member of the editorial board of
Figures


References
-
- Addeo A, Banna GL, Metro G, Di Maio M. Chemotherapy in combination with immune checkpoint inhibitors for the first‐line treatment of patients with advanced non‐small cell lung cancer: a systematic review and literature‐based meta‐analysis. Front Oncol. 2019;9:264. doi:10.3389/fonc.2019.00264 - DOI - PMC - PubMed
-
- Horinouchi H, Nishio M, Hida T, et al. Three‐year follow‐up results from phase II studies of nivolumab in Japanese patients with previously treated advanced non‐small cell lung cancer: pooled analysis of ONO‐4538‐05 and ONO‐4538‐06 studies. Cancer Med. 2019;8(11):5183‐5193. doi:10.1002/cam4.2411 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

